
The team of Fener Chen (an academician of CAE) and Huijing Wang (an associate research fellow) of the West China School of Pharmacy(WCSP) developed astereodivergent iodocyclization methodof nucleosides with the asymmetric iodine cyclization reaction of additive-controlled olefins as the key reaction, and completed the synthesis of anti-HIV agent stavudine as well as molnupiravir, an anti-SARS-C0V-2 agent. The research findings were published under the title of “Additive-Controlled Asymmetric Iodocyclization Enables Enantioselective Access to Both α- and β-nucleosides” in Nature Communications(Nat. Commun., 2023, 14, 138, DOI: 10.1038/s41467-022-35610-w.)The research was funded by the NationalNatural Science Foundation of China (Grant No. 22201192). Qi Wang, a Class 2020 doctoral student of WCSP is the first author, and Huijing Wang and Fener Chen are the co corresponding authors.
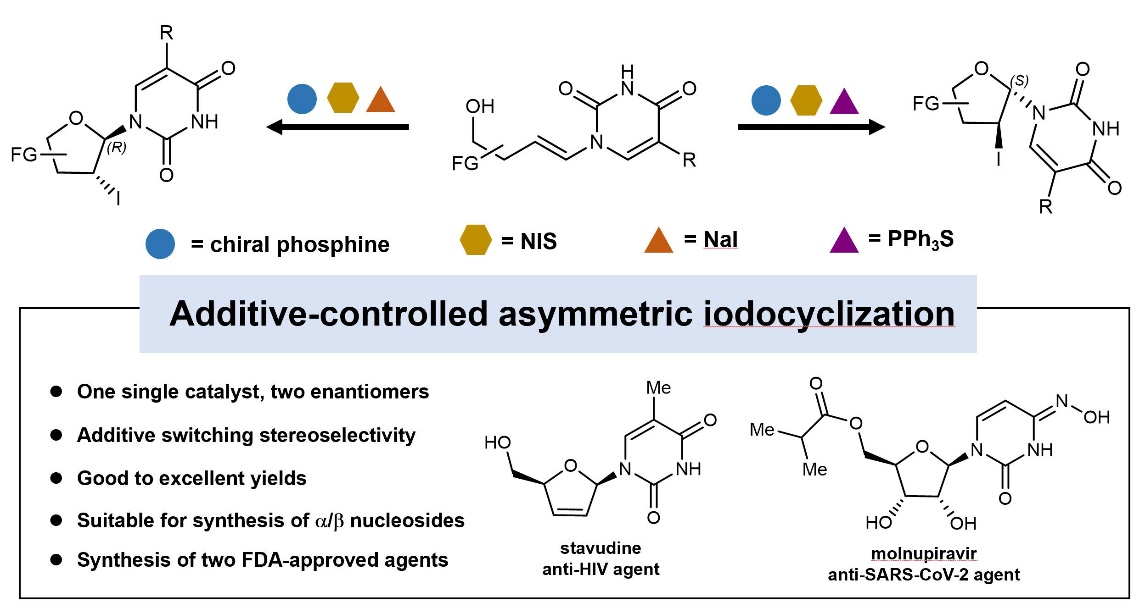
“β-Nucleosides and their analogs are dominant clinically-used antiviral and antitumor drugs. α-Nucleosides, the anomers of β-nucleosides, exist in nature and have significant potentialas drugs or drug carriers. Currently, the most widely used methods for synthesizing β- and α-nucleosides are via N-glycosylation and pentose aminooxazoline, respectively. However, the stereoselectivities of both methods highly depend on the assisting group at the C2’ position. Herein, we report an additive-controlled stereodivergent iodocyclization method for theselective synthesis of α- or β-nucleosides. The stereoselectivity at the anomeric carbon is controlled by the additive (NaI for β-nucleosides; PPh3S for α-nucleosides). A series of β- and α-nucleosides are prepared in high yields (up to 95%) and stereoselectivities (β:α up to 66:1, α:β up to 70:1). Notably, the introduced iodine at the C2’ position of the nucleoside is readily functionalized, leading to multiple structurally diverse nucleoside analogs, includingstavudine, an FDA-approved anti-HIV agent, and molnupiravir, an FDA-approved anti-SARS-CoV-2 agent.” (Abstract) The development of stereocontrollable and flexible synthesis methods is of great significance to the development of nucleoside drugs.
https://www.nature.com/articles/s41467-022-35610-w
