On Feb. 10, Prof. Zhenhua Shao’s team from the State Key Laboratory of Biotherapy(SKLB), West China Hospital (WCH) of Sichuan University published inCellan online research paper entitled “Ligand Recognition and Allosteric Regulation of DRD1-Gs Signaling Complexes”.As a joint research project with Profs. Xiao Yu and Jin-Peng Sun of Shandong University, Prof. Lei Zhang of Xi'an Jiaotong University and Prof. Yang Du of the Chinese University of Hong Kong (Shenzhen), the paper analyzes by means of freeze electron microscopy the three-dimensional structures of DRD1-GS in complex with catechol-based agonist (Fenoldopam, A77636 and SKF83959), non-catechol based agonist PW0464, happiness hormone dopamine and positive allosteric regulator LY3154207 for the first time. West China Hospital is the first work unit and corresponding unit, and SCU’s Associate Professor Wei Yan is the co first author.

Dopamine is a neurotransmitter that can bring pleasure to people and plays an important role in the treatment of central nervous system diseases.
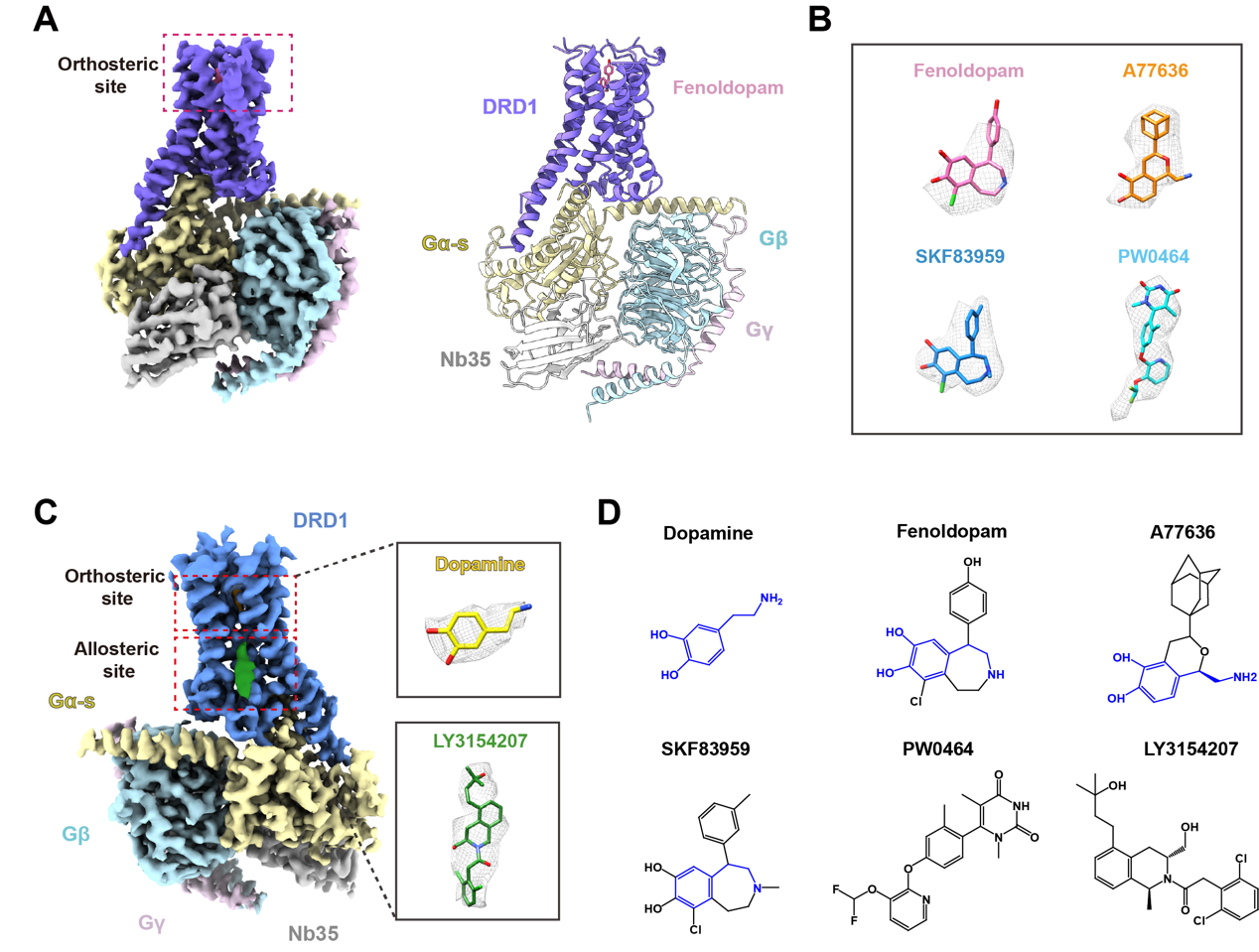
Figure 1. The cryoelectron microscopy (cryo-EM) structures of DRD1-Gs.
“Dopamine receptors, including D1- and D2-like receptors, are important therapeutic targets in a variety of neurological syndromes, as well as cardiovascular and kidney diseases. Here, we present five cryoelectron microscopy (cryo-EM) structures of the dopamine D1 receptor (DRD1) coupled to Gs heterotrimer in complex with three catechol-based agonists, a non-catechol agonist, and a positive allosteric modulator for endogenous dopamine. These structures revealed that a polar interaction network is essential for catecholamine-like agonist recognition, whereas specific motifs in the extended binding pocket were responsible for discriminating D1- from D2-like receptors. Moreover, allosteric binding at a distinct inner surface pocket improved the activity of DRD1 by stabilizing endogenous dopamine interaction at the orthosteric site. DRD1-Gs interface revealed key features that serve as determinants for G protein coupling. Together, our study provides a structural understanding of the ligand recognition, allosteric regulation, and G protein coupling mechanisms of DRD1.” (Summary)
The research paper highlights the following five points:
• Structures of DRD1-Gs in complex with catechol-based and non-catechol agonists
• Key polar interaction network is involved in DRD1 activation
• Specific features for agonists interaction and Gs coupling of DRD1 are determined
• Structure of DRD1-Gs complexed with dopamine and positive allosteric modulator
• Structural basis of allosteric regulation of DRD1 by LY3154207 is investigated
In conclusion, the research team has analyzed the structures of the complex of dopamine receptor DRD1 and G protein by means of single particle freeze electron microscopy, thus explaining in detail the ligand recognition, allosteric regulation and coupling mechanism of DRD1 with G protein at the atomic level. This research brings new hope for the drug development and treatment of hypertension, Parkinson's syndrome, kidney injury and so forth.
Profs. Ping Fu and Shengyong Yang of theDivision of Nephrology and Kidney Research Institute, State Key Laboratory of Biotherapy and Cancer Center, WCH,Prof. Huanxiang Liu of Lanzhou University, and Prof. Lizhe Zhu of the Chinese University of Hong Kong (Shenzhen) were co authors of the paper. The experimental work of this research has been completed in China, and supported by the key R & D program of Sichuan Provincial Department of Science and Technology, the key R & D program of the Ministry of Science and Technology, and the National Natural Science Foundation of China.
