The research team of Prof. Yuan Wang (State Key Laboratory of Biotherapy and Cancer Center)( SKLBCC), Prof. Lu Chen (West China Second University Hospital) and Prof. Yan Zhang (National Clinical Research Center For Geriatrics) has published in Cell Research a paper titled “Sequential Fate-switches in Stem-like Cells Drive the Tumorigenic Trajectory from Human Neural Stem Cells to Malignant Glioma”. Xiaofei Wang and Ran Zhou, doctoral students of SKLBCC in our university, are the first authors of the paper. The State Key Laboratory of biotherapy / Department of Neurology/ Department of Neurosurgery, West China Hospital(WSH) are the first author and corresponding author work unit.
“Glioblastoma (GBM) is an incurable and highly heterogeneous brain tumor, originating from human neural stem/progenitor cells (hNSCs/hNPCs) years ahead of diagnosis. Despite extensive efforts to characterize hNSCs and end-stage GBM at bulk and single-cell levels, the de novo gliomagenic path from hNSCs is largely unknown due to technical difficulties in early-stage sampling and preclinical modeling. Here, we established two highly penetrant hNSC-derived malignant glioma models, which resemble the histopathology and transcriptional heterogeneity of human GBM.” (Abstract)
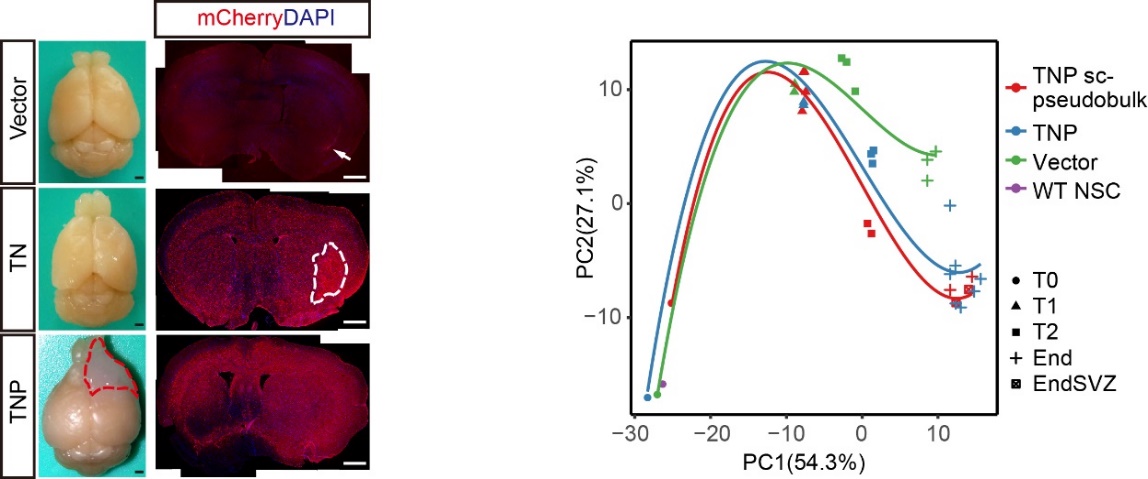
“Integrating time-series analyses of whole-exome sequencing, bulk and single-cell RNA-seq, we reconstructed gliomagenic trajectories, and identified a persistent NSC-like population at all stages of tumorigenesis. Through trajectory analyses and lineage tracing, we showed that tumor progression is primarily driven by multi-step transcriptional reprogramming and fate-switches in the NSC-like cells, which sequentially generate malignant heterogeneity and induce tumor phenotype transitions. We further uncovered stage-specific oncogenic cascades, and among the candidate genes we functionally validated C1QL1 as a new glioma-promoting factor. Importantly, the neurogenic-to-gliogenic switch in NSC-like cells marks an early stage characterized by a burst of oncogenic alterations, during which transient AP-1 inhibition is sufficient to inhibit gliomagenesis. Together, our results reveal previously undercharacterized molecular dynamics and fate choices driving de novo gliomagenesis from hNSCs, and provide a blueprint for potential early-stage treatment/diagnosis for GBM.” (Abstract)

In general, this study has established a new and efficient modeling method, and showed the malignant trajectory from human neural stem cells to GBM in a panoramic way. It is of great significance with regard to understanding the pathogenesis and heterogeneity of GBM, thus, developing early clinical diagnosis and treatment strategies on this basis. The researchis supported by the National Key Research and Development Program of China, Stem Cell and Translational Research (2017YFA0106500), the Distinguished Young Scientists Program of Sichuan Province (2019JDJQ0029), and the 135 Program for Excellent Scholars at West China Hospital (ZYYC20019).
Article link:https://doi.org/10.1038/s41422-020-00451-z
