On Nov. 16, 2020, Nature Structural & Molecular Biology published a research paper entitled“Structural Insights into Outer Membrane Asymmetry Maintenance in Gram-negative Bacteria by MlaFEDB”by Prof. Haohao Dong’s team of the State Key Laboratory of Biotherapy and Cancer Center, SCU. The research work was conducted in collaboration with the team of Prof. Xing Zhang, director of the Center of Cryo-Electron Microscopy, Zhejiang University(ZJU); and Prof. Changjiang Dong’s team of Biomedical Research Centre, Norwich Medical School, University of East Anglia. The first authors are Dr. Xiaodi Tang, associate professor of the State Key Laboratory of Biotherapy and Cancer Center, SCU; Dr. Shenghai Chang of the Center of Cryo-Electron Microscopy, ZJU and Wen Qiao, a doctoral student of SCU. SCU’s State Key Laboratory of Biotherapy and Cancer Center and the National Clinical Research Center for Geriatrics are the corresponding work units.
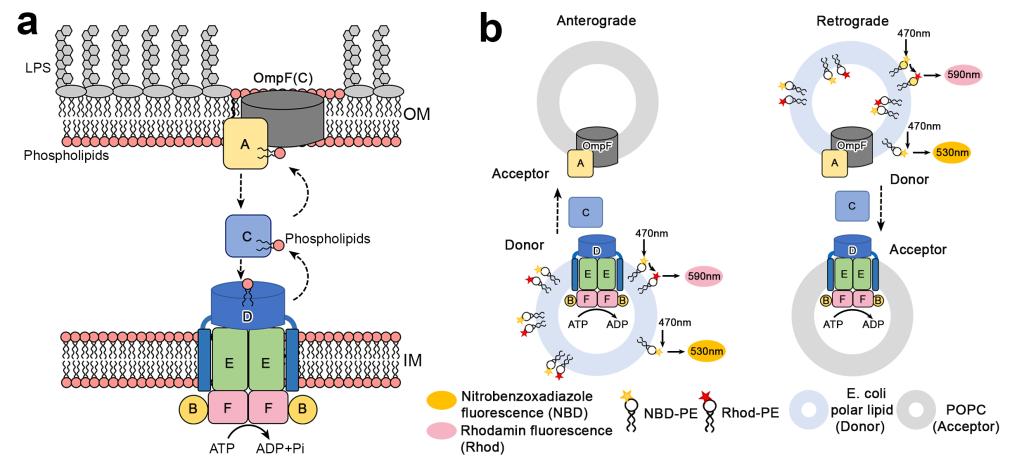
Fig. 1: PL transport between OM and IM phospholipids by the Mla pathway.
This study has revealed four high resolution freeze electron microscopic structures of MlaFEDB, a protein mechanical complex used by multidrug-resistant bacteria and Gram-negative bacteria, to transport phospholipid components in outer membrane and maintain asymmetric structure of outer membrane.
“The highly asymmetric outer membrane of Gram-negative bacteria functions in the defense against cytotoxic substances, such as antibiotics. The Mla pathway maintains outer membrane lipid asymmetry by transporting phospholipids between the inner and outer membranes. It comprises six Mla proteins, MlaFEDBCA, including the ABC transporter MlaFEDB, which functions via an unknown mechanism. Here we determine cryo-EM structures of Escherichia coli MlaFEDB in an apo state and bound to phospholipid, ADP or AMP-PNP to a resolution of 3.3–4.1 Å and establish a proteoliposome-based transport system that includes MlaFEDB, MlaC and MlaA–OmpF to monitor the transport direction of phospholipids.”(Abstract)
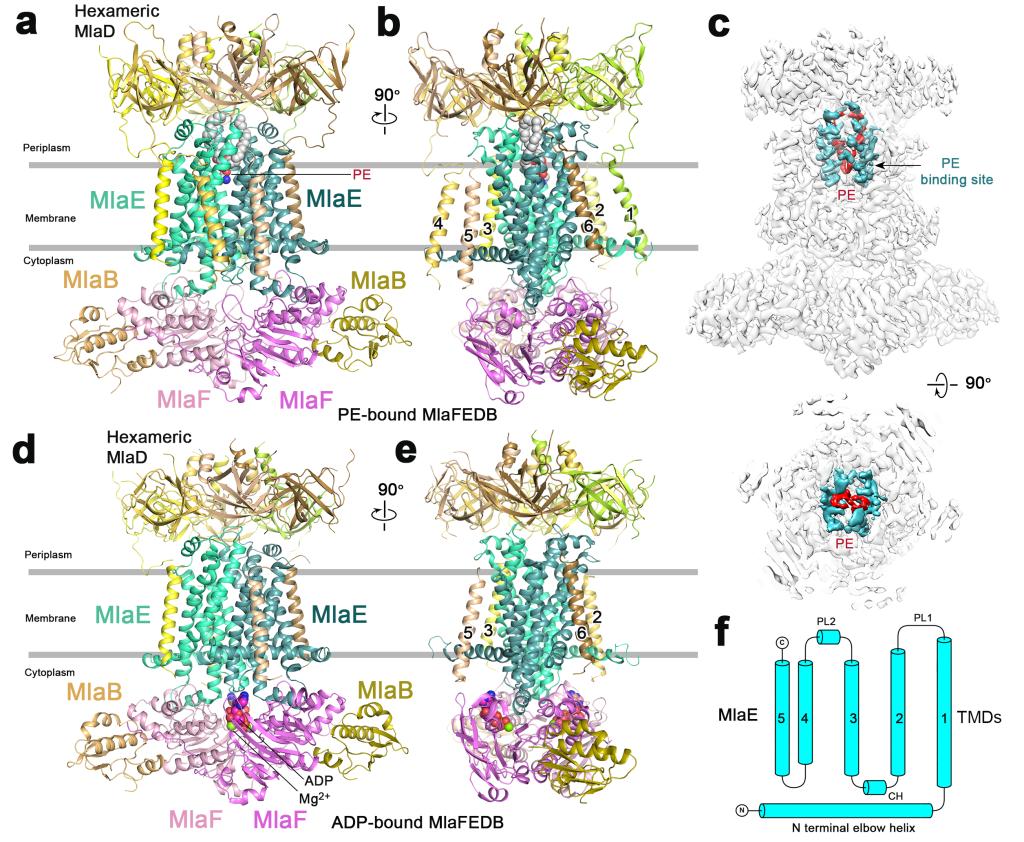
Fig. 2: Architectures of PE- and ADP-bound MlaFEDB complexes.
“In vitro transport assays and in vivo membrane permeability assays combined with mutagenesis identify functional residues that not only recognize and transport phospholipids but also regulate the activity and structural stability of the MlaFEDB complex. Our results provide mechanistic insights into the Mla pathway, which could aid antimicrobial drug development.” (Abstract)
In this study, a number of functional sites were identified to improve bacterial drug sensitivity through mutation and transport experiments. These functional sites can be used as effective targets for the design of new antibiotics. In conclusion, this study opens up a new direction for the research and development of new drugs against super bacterial infection, and has important guiding significance.
Article Link:https://www.nature.com/articles/s41594-020-00532-y
