Led by Sichuan University and in cooperation with researchers from Nanjing Forestry University, West Virginia University and Texas Tech University, the researchers of SCU’s College of Life Sciences(CLS) published online in Molecular Biology and Evolution a research paper entitled “A General Model to Explain Repeated Revolutions of Sex Determination in the Salicaceae. In this paper, a universal molecular genetics model for frequent sex system transformation in Salicaceae was proposed. The first authors are Wenlu Yang, a doctoral student of CLS, and Deyan Wang, Yiling Li, Zhiyang Zhang, master students of CLS. The corresponding authors are CLS professors Tao Ma and Jianquan Liu, Professor Stephen P. DiFazio of West Virginia University and Professor Matthew Olson of Texas Tech University. The research was funded by the National Natural Science Foundation of China, and the major research project program of the Ministry of Science and Technology.
The origin and evolution of both sexes has always been one of the most interesting topics for biologists. There are two common sex determining systems in organisms, XY system. Male is composed of two heteromorphic chromosomes, and female is composed of two homomorphic sex chromosomes, such as human beings. On the contrary, ZW system is composed of two homozygous chromosomes, and female consists of two heteromorphic chromosomes, such as birds and fish. Almost all Salicaceae plants are dioecious, and their sex chromosomes have no obvious morphological differences and are in the early stage of evolution. Studies indicate that there is a wide diversity of sex determination systems in Salicaceae, and their sex determination regions are always in different regions of the same chromosome (or between different chromosomes), and jump repeatedly in a very short evolutionary time, resulting in two major sex determination systems (XY / ZW). Its molecular genetic model has become an important window for understanding the origin and evolution of sex chromosomes. The genome-wide association studies (GWAS) of P. euphratica and P. alba revealed that the sex determining region of P. euphratica was located in the near telomere region of chromosome 14, which was XY type sex determination system; whereas the sex determining region of P. Alba was located in the near centromere region of chromosome 19, and the sex determination system was ZW type. By assembling, annotating and comparing the sex determining regions of the two species, it was found that there were homologous sequences encoding type-A cytokinin response regulator (RR) on the sex chromosomes of the two species. Among them, there were three complete RR genes on the W chromosome of Populus alba and multiple reverse repeats of RR fragments on the Y chromosome of Populus euphratica. Therefore, it is speculated that the reverse repeat structure of RR fragment on Y chromosome of Populus euphratica is likely to inhibit its expression by producing siRNA and mediating the methylation of complete RR gene on its autosomal, which is also confirmed by small RNA sequencing data.
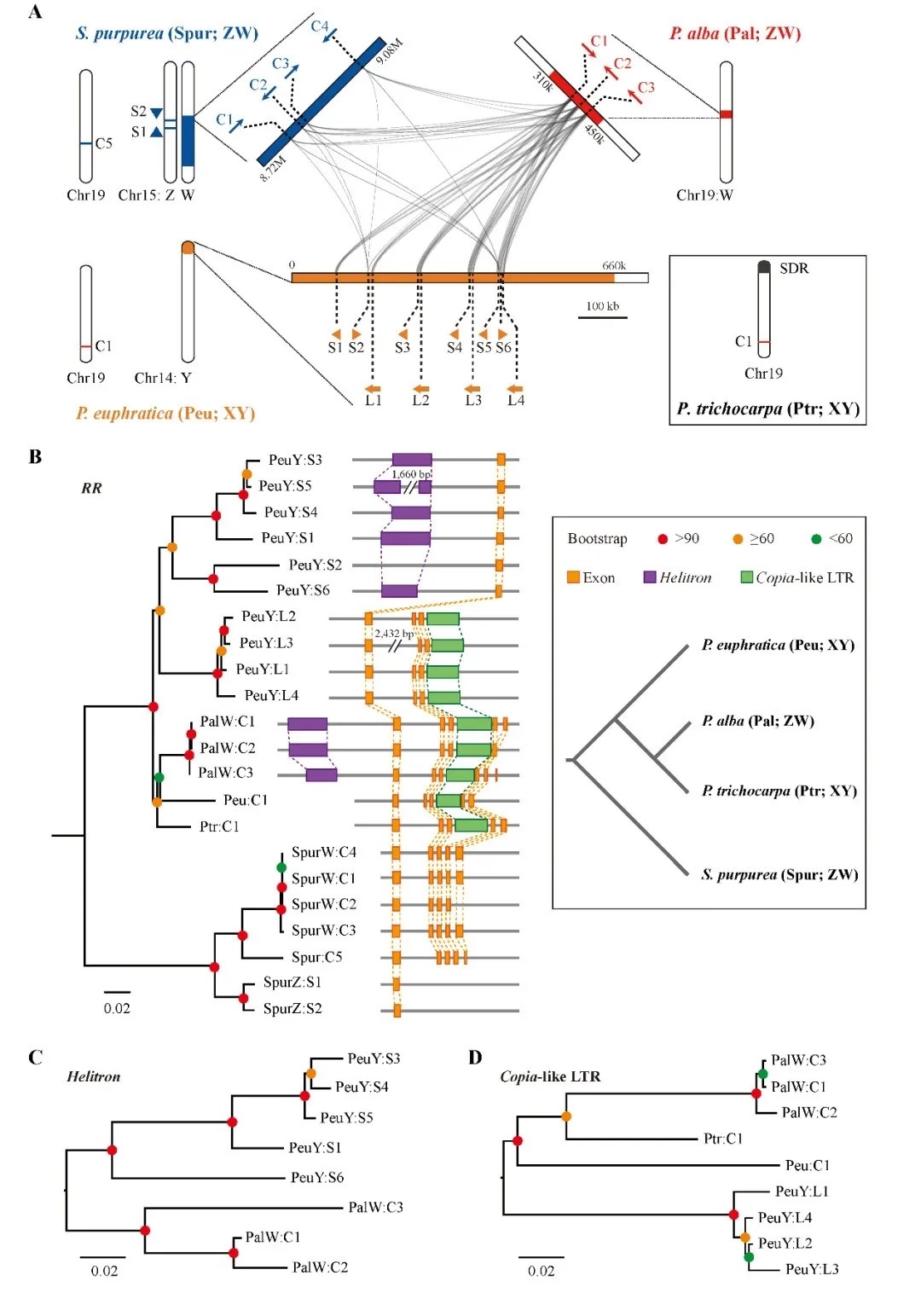
“Dioecy, the presence of separate sexes on distinct individuals, has evolved repeatedly in multiple plant lineages. However, the specific mechanisms by which sex systems evolve and their commonalities among plant species remain poorly understood.--- Our results reveal an XY system of sex determination on chromosome 14 of P. euphratica, and a ZW system on chromosome 19 of P. alba. We further assembled the corresponding sex determination regions, and found that their sex chromosome turnovers may be driven by the repeated translocations of a Helitron-like transposon. During the translocation, this factor may have captured partial or intact sequences that are orthologous to a type-A cytokinin response regulator gene. Based on results from this and other recently published studies, we hypothesize that this gene may act as a master regulator of sex determination for the entire family. We propose a general model to explain how the XY and ZW sex systems in this family can be determined by the same RRgene. Our study provides new insights into the diversification of incipient sex chromosomes in flowering plants by showing how transposition and rearrangement of a single gene can control sex in both XY and ZW systems.” (Abstract)
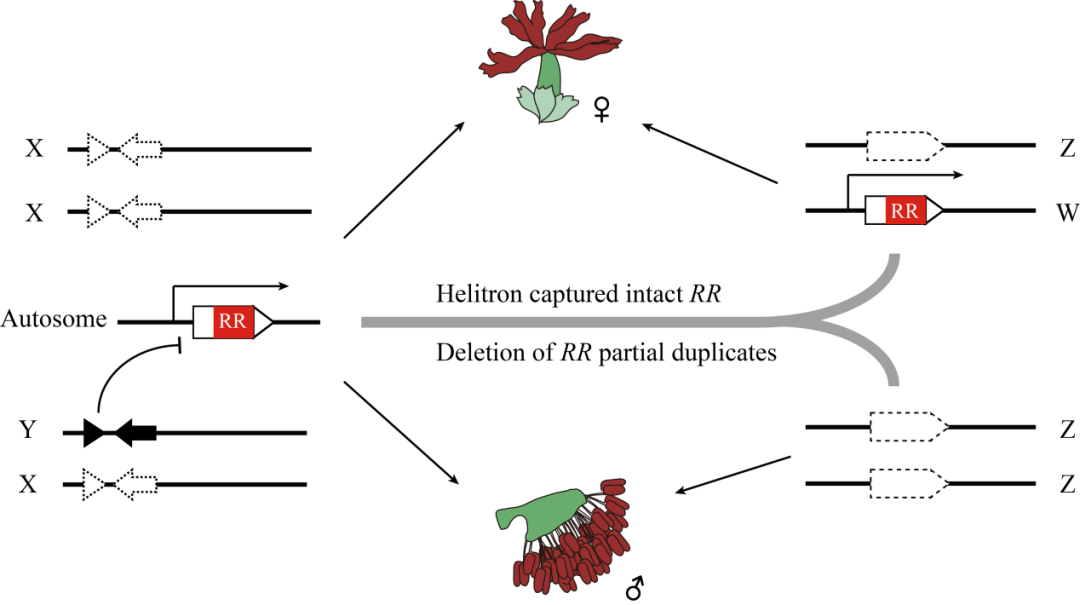
In conclusion, this study provides a new insight into the origin and diversity of sex chromosomes in flowering plants, and lays a theoretical foundation for further study on the molecular mechanism of sex determination and evolution of sex chromosomes in Salicaceae plants. It also provides a reference basis for the study of sex determination mechanism in other dioecious plants.
Article link: https://doi.org/10.1093/molbev/msaa261
