An important progress has been made recently in the research on lithium- and potassium-ion batteries as a result of the cooperative project among Professor Yun Zhang and research fellow Hao Wu’s research team; Mr. Shixue Dou, fellow of Australian Academy of Engineering and professor of University of Wollongong; and Professor Qiaobao Zhang of Xiamen University.The article “Realizing Reversible Conversion-Alloying of Sb ( V ) in Polyantimonic Acid for Fast and Durable Lithium- and Potassium-ion Storage” is published as a cover story in Advanced Energy Material ( IF=24.884 ).
The first author of this paper is Boya Wang, a Class 2017 doctoral student from the SCU College of Materials Science and Engineering. Besides, four Class 2016 undergraduate students Zhiwen Deng, Yuting Xia, Jiaxuan Hu, and Hongju Li have participated in the research. Zhiwen Deng and Yuting Xia have been recommended to a master’s program at Tsinghua University in 2020 whereas Jiaxuan Hu and Hongju Li to a master’s program in Westlake University in 2020. The corresponding authors are research fellow Hao Wu and Professor Yun Zhang, and Sichuan University is the sole corresponding work unit of this paper.


Figure 1. The Cover illustration: the composite of nano-octahedrapolyantimonic acid and graphene realizes the fast and durable lithium- and potassium-ion storage.
In recent years, researchers have developed numerous electrode materials to store alkali-metal ions ( such as Li + and K + ions ) in secondary batteries. However, only a few electrode materials can be used as a general main material for the storage of alkali metal ions. Polyantimonic Acid ( PAA, h2sb2o6 · nH2O, 0 ≤ n ≤ 4 ) is an important antimony based compound. Though it is widely used in the preparation of fireproof materials, sensors and ion exchange agents, it has not been used in energy storage and conversion. Because of its three-dimensional interconnected and its tunnel-like pyrochlore crystal structure, polyantimonic acid ( PAA ) is very promising as a high capacity electrode material for electrochemical storage of alkali metal ions. However, due to its extremely low electronic conductivity ( ~ 10-10scm-1 ), it is still a challenge to prepare electrochemical reversible polyantimonic acid electrode materials for electrochemical energy storage, and has never been reported.
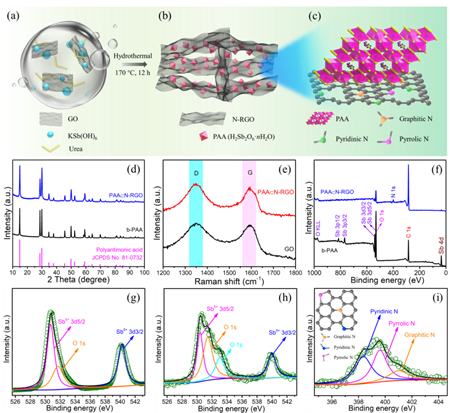
Figure 2. Preparation process and structure characterization of composite
“Combining nanostructure engineering with a conductive‐network construction strategy, here is reported a facile one‐pot synthesis protocol for crafting uniform internal‐void‐containing PAA nano‐octahedra in a composite with nitrogen‐doped reduced graphene oxide nanosheets ( PAA⊂N‐RGO ), and for the first time, realizing the reversible storage of both Li+ and K+ ions in PAA⊂N‐RGO. Such an architecture, as validated by theoretical calculations and ex/in situ experiments, not only fully takes advantage of the large‐sized tunnel transport pathways ( 0.37 nm2 ) of PAA for fast solid‐phase ionic diffusion but also leads to exponentially increased electrical conductivity ( 3.3 S cm−1 in PAA⊂N‐RGO vs 4.8 × 10−10 S cm−1 in bare‐PAA ) and yields an inside‐out buffer function for accommodating volume expansion. Compared to electrochemically irreversible bare‐PAA, PAA⊂N‐RGO manifests reversible conversion‐alloying of Sb ( V ) toward fast and durable Li+‐and K+‐ion storage.” ( Abstract )
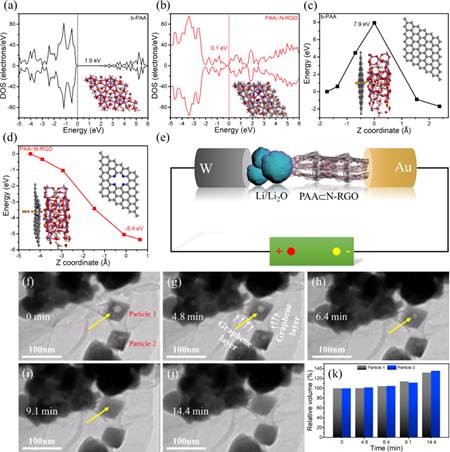
Figure 3. Theoretical calculation and in situ TEM characterization
This study not only shows the successful application of polyantimonic acid as a high capacity electrode material in lithium- and potassium-ion batteries, but also lays an important research foundation for its further development and application in other types of secondary batteries.
Article link:https://onlinelibrary.wiley.com/doi/10.1002/aenm.201903119
