The "Transcatheter Aortic Valve System" developed by Professor Chen Mao's team from the Department of Cardiology at West China Hospital has been approved for market by the National Medical Products Administration. As the first domestic balloon expandable transcatheter aortic valve product, this system can greatly reduce the economic burden for patients with heart valve disease, and also marks that China's treatment level for heart valve disease has once again reached the international advanced level.
This system was developed after eight years of research by Professor Chen Mao's team in collaboration with Shanghai's medical technology enterprises. It is suitable for patients aged 70 years or older who are diagnosed with symptomatic, calcified, severe degenerative aortic valve stenosis, and are not suitable for routine surgical valve replacement.

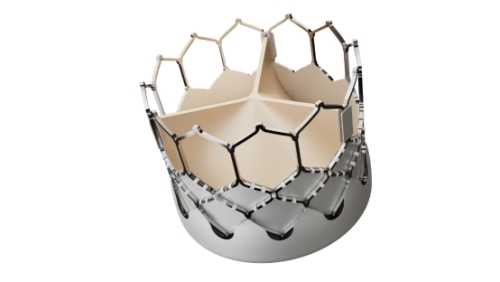
The system consists of a transcatheter aortic valve, a transcatheter aortic valve delivery system (including a delivery device and valve loading device), an aortic valve balloon dilation catheter, a clamping device, and a balloon inflation device. Under the monitoring of medical imaging equipment, this product is implanted into the aortic valve annulus of the human body through a catheter via the femoral artery, replacing the original diseased aortic valve, improving stenosis in the affected area, and enhancing cardiac function.
Being benchmarked against the latest international valve expansion technology, the system has adopted a short stent valve design with ball expansion, and is innovative in various aspects such as valve frame, valve skirt, and delivery system. Its disc holder is made of cobalt chromium alloy, and the radial support force generated by it can compress the residual disc to a smaller size. The unique biological tissue processing technology can further improve the service life of the valve. In addition, the design of the inner and outer sealing membrane skirt can effectively reduce valve leakage around the valve. The delivery system has superior bending performance, which improves the passage of the aortic arch during surgery, effectively reduces damage to the vascular wall, and achieves precise positioning.
Drawing upon the "Joint Laboratory of Biomaterials and Medical Device Technology Innovation", Chen Mao's team has fully leveraged the advantages of clinical technology and research foundation in biomaterials at West China Hospital. The team joined efforts with the National Engineering Research Center for Biomaterials and conducted multi-center clinical trials in the heart centers of nine large tertiary hospitals across the country, including the West China Hospital. The researchers have eventually developed the first balloon expandable interventional aortic valve, filling the gap in this field in China.
