
In collaboration with Professor Yun-Dong Wuof Peking University Shenzhen Graduate School,the team of Professor Dawen Niu from the State Key Laboratory of Biotherapy and Cancer Center(SKLB),West China Hospital, and School of Chemical Engineering, published in Nature a research paper entitled “Catalytic Glycosylation for Minimally Protected Donors and Acceptors" on June 17. The researchers developed a universal platform forsimultaneous control of site-, stereo-,selective O-glycosylation reactions between unprotected or poorly protected donors and acceptors.
Sichuan University is the first corresponding work unit, with the doctoral students of Qiu-Di Dang from the SKLB and Yi-Hui Deng from the Peking University Shenzhen Graduate School as co first authors, and Dawen Niu and Yun-Dong Wu as co corresponding authors.
"Oligosaccharides have myriad functions throughout biology.1,2To investigate these functions requires multi-step chemical synthesis of these structurally complex molecules. With a dense concentration of stereocentres and hydroxyl groups, oligosaccharide assembly throughO-glycosylation requires simultaneous control of site-, stereo-, and chemoselectivities3,4. Chemists have traditionally relied on protecting group manipulations for this purpose,5–8adding a lot of synthetic work. Here, we report a glycosylation platform that enables selective coupling between unprotected or minimally protected donor and acceptor sugars, producing 1,2-cis-O-glycosides in a catalyst-controlled, site-selective manner. Radical-based activation9of allyl glycosyl sulfones forms glycosyl bromides. A designed aminoboronic acid catalysts bring this reactive intermediate close to an acceptor through a network of noncovalent hydrogen bonding and reversible covalent B–O bonding interactions, allowing precise glycosyl transfer. The site of glycosylation can be switched with different aminoboronic acid catalysts by affecting their interaction modes with substrates. The method accommodates a wide range of sugar types, amenable to preparing naturally occurring sugar chains and pentasaccharides containing 11 free hydroxyls. Experimental and computational studies provide insights into the origin of selectivity outcomes." (Abstract)
The research work was funded by the National Natural Science Foundation of China's Excellent Youth Program and Innovation Group Program, Sichuan University Research Institute Innovation Leading Talent Program, and Sichuan University West China Hospital's 1-3-5 Plan Project. Part of the experimental analysis and testing were completed with the help of Prof. Xiaoyan Wang of the Analytical and Testing Center, Sichuan University and Prof. Xihua Wang from the Mass Spectrometry of the College of Life Sciences, Sichuan University.
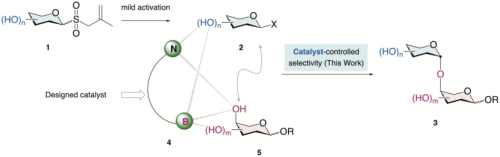
Fig. a) Background of oligosaccharide synthesis. b) Reaction design
Source: https://www.nature.com/articles/s41586-024-07695-4
