Lanthipeptides are an important group of natural products of ribosomal peptides produced by microorganisms. They have a variety of structural and biological characteristics, including anti-bacterial, anti-virus, anti-pain, treatment of cystic fibrosis and so on. These compounds have a unique mechanism of action and rarely produce drug resistance. They are an important source of active drug research and development. Immature lanthipeptide (precursor peptide) is composed of N-terminal leading peptide and C-terminal core peptide. The removal of leading peptide is very important for the maturation and biological activity of wool thiopeptide. LanPM1 is a zinc ion dependent metallopeptidase that can remove leading peptides. This kind of enzyme has a good potential for the production of wool thiopeptide compounds in vitro.

In collaboration with research teams from Nanjing University, Professor Rui Bao’s team of theCenter of Infectious Diseases, State Key Laboratory of Biotherapy, West China Hospital, Sichuan University, published in Nature Chemical Biology the latest research findings in a paper entitled“Conformational remodeling enhances activity of lanthipeptide zinc-metallopeptidases”
Taking EryP, a member of LanPM1 family, as the starting point, the study analyzed the high-resolution crystal structure of lanthipeptide zinc- metallopeptidase EryP with various conformations. Combined with molecular dynamics simulation and in vitro enzyme activity, it revealed the unique catalytic mechanism of LanPM1 and significantly improved the catalytic efficiency of LanPM1 based on structural modification (Fig. 1).
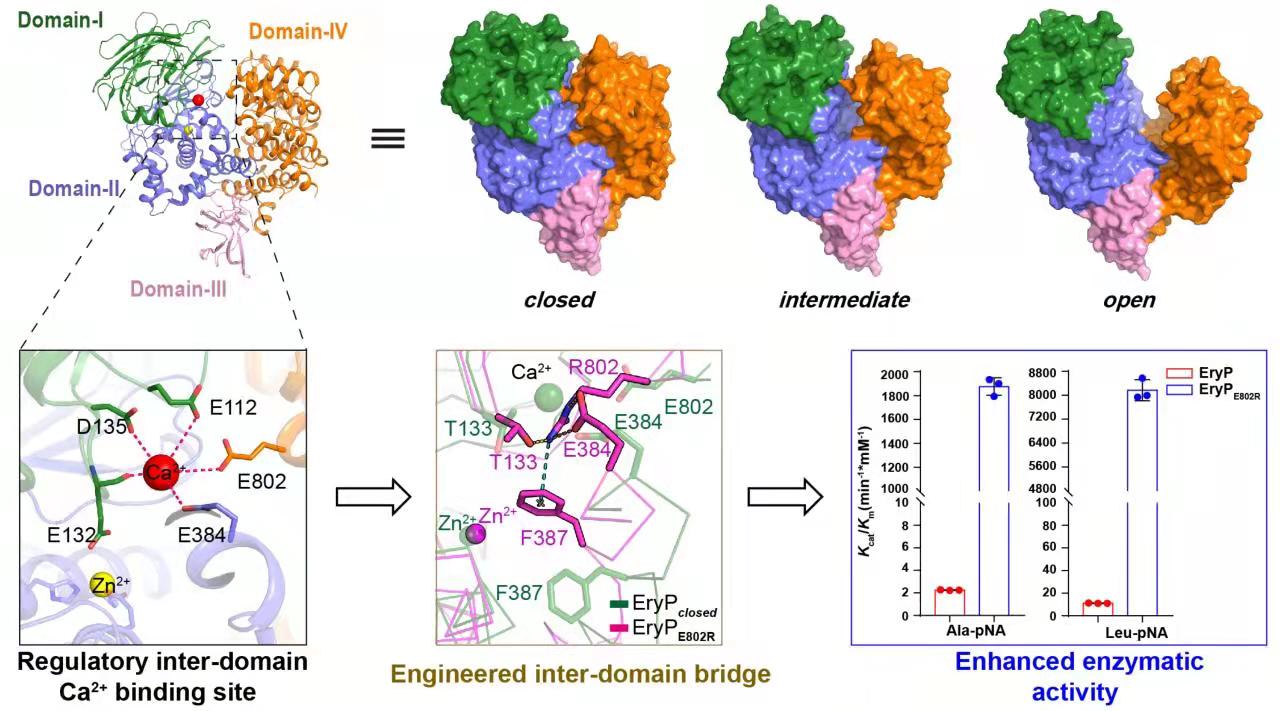
Fig.1 Overall structure and the metal ion binding sites of EryP.
“Lanthipeptides are an important group of natural products with diverse biological functions, and their biosynthesis requires the removal of N-terminal leader peptides (LPs) by designated proteases. LanPM1 enzymes, a subgroup of M1 zinc-metallopeptidases, have been recently identified as bifunctional proteases with both endo- and aminopeptidase activities to remove LPs of class III and class IV lanthipeptides. Herein, we report the biochemical and structural characterization of EryP as the LanPM1 enzyme from the biosynthesis of class III lanthipeptide erythreapeptin. We determined X-ray crystal structures of EryP in three conformational states, the open, intermediate and closed states, and identified a unique interdomain Ca2+ binding site as a regulatory element that modulates its domain dynamics and proteolytic activity. Inspired by this regulatory Ca2+binding, we developed a strategy to engineer LanPM1 enzymes for enhanced catalytic activities by strengthening interdomain associations and driving the conformational equilibrium toward their closed forms.”
TheCenter of Infectious Diseases, State Key Laboratory of Biotherapy, West China Hospital, Sichuan University is the first work unit of this paper. The co first authors are Chang Zhao, a doctoral student of Sichuan University, and Wangjian Sheng, a doctoral graduate of Nanjing University, and the corresponding authors are Prof. Huan Wang and Dr. Wanqing Wei of Nanjing University and Prof. Rui Bao.This work is supported by the National Science Foundation of China, the Natural Science Foundation of Jiangsu Province and the West China Hospital, Sichuan University, etc.
Rui Bao's team has long studied the structure and function of important proteins in bacterial stress resistance, pathogenesis and metabolism. The team has made numerous significant findings in pili assembly machine, virulence factors synthesis, bacterial channel protein and so on. Relevant works have been published in Nature Chemical Biology, Biosensors & Bioelectronics,Proceedings of the National Academy of Sciences,PLoS Pathogens, and mBio.
https://www.nature.com/articles/s41589-022-01018-2
