
Prof. Dawen Niu's team from the State Key Laboratory of Biotherapy (SKLB) of West China Hospital/School of Chemical Engineering published an article titled "Palladium Catalysis Enables Cross-Coupling - Like SN2-Glycolysis of Phenols" in Science on November 24. Lifan Deng, a doctoral student of the SKLB, and Dr. Yingwei Wang from theDepartment of Nuclear Medicine & Laboratory of Clinical Nuclear Medicine,are the co first authors of the article, and Dawen Niu is the corresponding author.
Glycosylation is a commonly used tool in drug development. The introduction of sugar units often changes the water solubility, targeting, metabolic stability, and other properties of target molecules. However, chemical methods for introducing sugar units into target molecules still face many challenges. " Deng et al. developed a versatile palladium-catalyzed reaction for the glycosylation of phenols that resembles palladium-catalyzed aryl carbon–oxygen cross-coupling reactions.Palladium oxidative addition to an easily prepared ortho-iodobiphenyl S-glycoside yields a complex that reacts with a wide range of phenolates through a SN2 mechanism to afford the glycosylated phenols with inversion of stereochemistry. This reaction works well with, but it is not limited to, 2-deoxy sugars and can be performed in one pot with other palladium-catalyzed cross-couplings to yield complex O-glycosides." (Editor's summary)
"Despite their importance in life and material sciences, the efficient construction of stereo-defined glycosides remains a challenge. Studies of carbohydrate functions would be advanced if glycosylation methods were as reliable and modular as palladium (Pd)-catalyzed cross-coupling. However, Pd-catalysis excels in forming sp2-hybridized carbon centers whereas glycosylation mostly builds sp3-hybridized C–O linkages. We report a glycosylation platform through Pd-catalyzed SN2 displacement from phenols toward bench-stable, aryl-iodide–containing glycosyl sulfides. The key Pd(II) oxidative addition intermediate diverges from an arylating agent (Csp2electrophile) to a glycosylating agent (Csp3electrophile). This method inherits many merits of cross-coupling reactions, including operational simplicity and functional group tolerance. It preserves the SN2 mechanism for various substrates and is amenable to late-stage glycosylation of commercial drugs and natural products." (Abstract)
The project was entirely completed through collaboration within Sichuan University. Theresearch work was funded by the National Natural Science Foundation of China's Excellent Youth Program and Innovation Group Project, the West China Hospital's 1.3-5 Plan Project, and the Innovation Leading Talent Project of theOffice of Scientific Research and Development. Prof. Jinsong Yang of Sichuan University and Prof. Peiyuan Yu from Southern University of Science and Technology provided valuable feedback on this study.
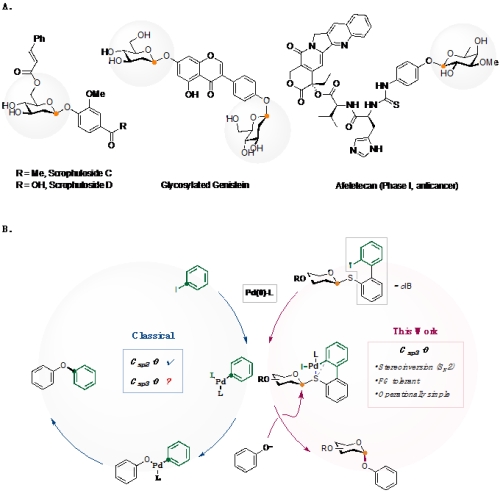
Figure A. Glycosylated phenols in drugs and natural products. Figure B. Reaction design.
https://www.science.org/doi/10.1126/science.adk1111
