
In collaboration with Professor Xing Zhang from the Center of Cryo Electron Microscopy of Zhejiang University, and Professor Ruhong Zhou from the College of Life Sciences of Zhejiang University, Haohao Dong, a research fellow, and Associate Professor Xiaodi Tang from the State Key Laboratory of Biotherapy and Cancer Center of West China Hospital, published a research paper titled “Structural Basis of BAM-Mediated Outer Membrane β-Barrel Protein Assembly” in Nature on April 26. The first authors are Chongrong Shen, a graduate student in the State Key Laboratory of Biotherapy of Sichuan University, Dr. Shenghai Chang, an engineer in the Center of Cryo Electron Microscopy of Zhejiang University School of Medicine, Qinghua Luo, a postdoctoral fellow of SCU, and Jun Chen, a postdoctoral fellow of the College of Life Sciences of Zhejiang University. The State Key Laboratory of Biotherapy/National Clinical Research Center for Geriatrics,West China Hospital is the work unit of the first authors and corresponding authors of this paper.
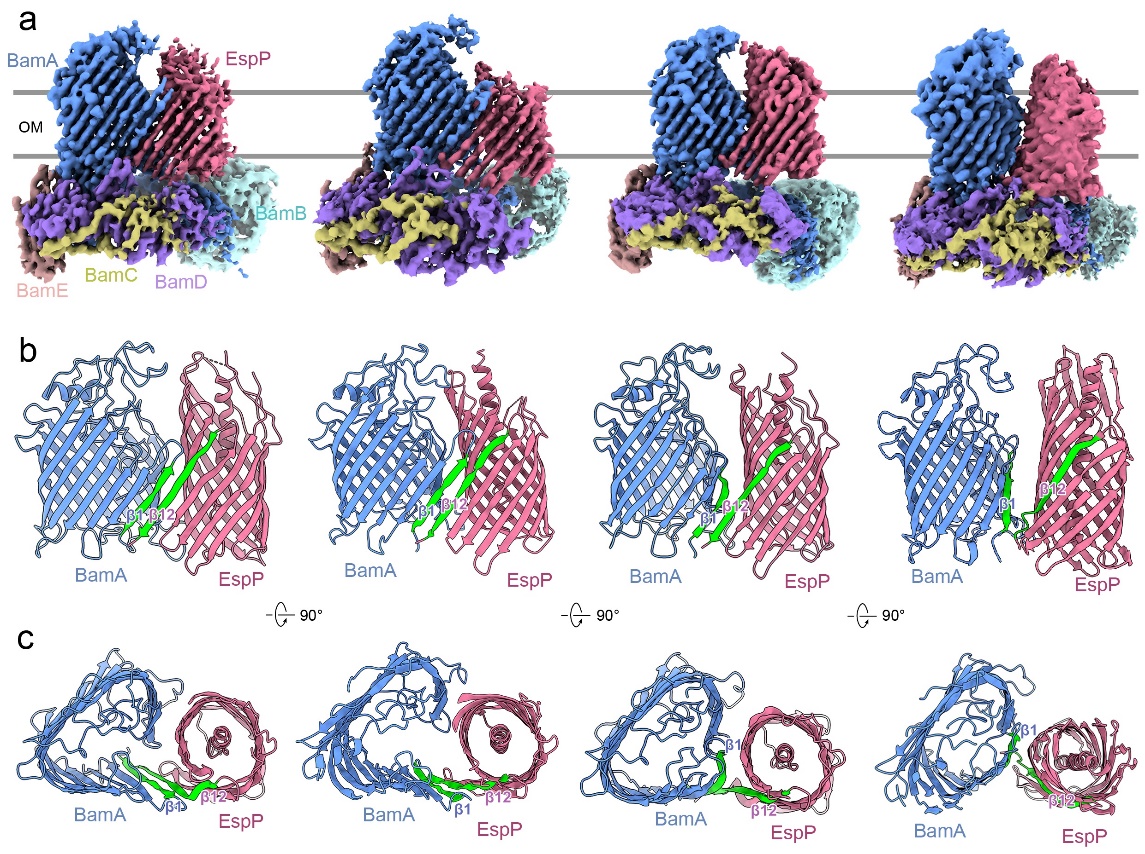
Fig. 1: Cryo-EM structures of intermediate assembly complexes of BAM and the substrate EspP.
This study first captured multiple intermediate conformations of BAM and the substrate EspP during the outer membrane folding and integration process (Figure 1). “The outer membrane structure is common in Gram-negative bacteria, mitochondria and chloroplasts, and contains outer membrane β-barrel proteins (OMPs) that are essential interchange portals of materials. All known OMPs share the antiparallel β-strand topology, implicating a common evolutionary origin and conserved folding mechanism. Models have been proposed for bacterial β-barrel assembly machinery (BAM) to initiate OMP folding;however, mechanisms by which BAM proceeds to complete OMP assembly remain unclear. Here we report intermediate structures of BAM assembling an OMP substrate, EspP, demonstrating sequential conformational dynamics of BAM during the late stages of OMP assembly, which is further supported by molecular dynamics simulations. Mutagenic in vitro and in vivo assembly assays reveal functional residues of BamA and EspP for barrel hybridization, closure and release. Our work provides novel insights into the common mechanism of OMP assembly.” (Abstract)
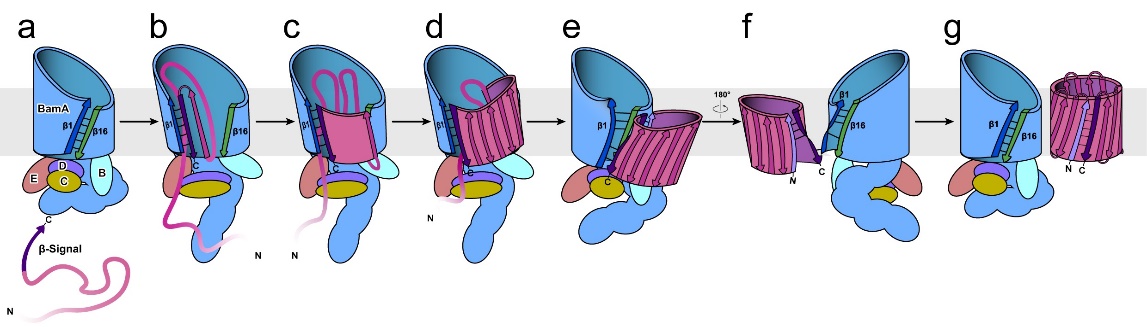
Fig. 5: Schematic diagram showing the proposed assembly mechanism of BAM.
The research findings were also published in a research briefing titled "Step-by-Step Assembly of a B-Barrier Protein in a Bacterial Membrane" in the same issue of Nature. Having been completed in China,this work was partially supported by the National Key Research and Development Program of China, the National Natural Science Foundation of China, the National Center of Technology Innovation for Biopharmaceuticals, the Fundamental Research Funds for Central Universities, and so forth.
https://www.nature.com/articles/s41586-023-05988-8
