
In collaboration with Prof. Ping Fu’s team, the research team of Zhenhua Shao (research fellow) and Wei Yan (an associate research fellow) at the Division of Nephrology and Kidney Research Institute, West China Hospital has published online in Cell Research their latest research achievements“Mechanism of Activation and Biased Signaling in Complement Receptor C5aR1”.
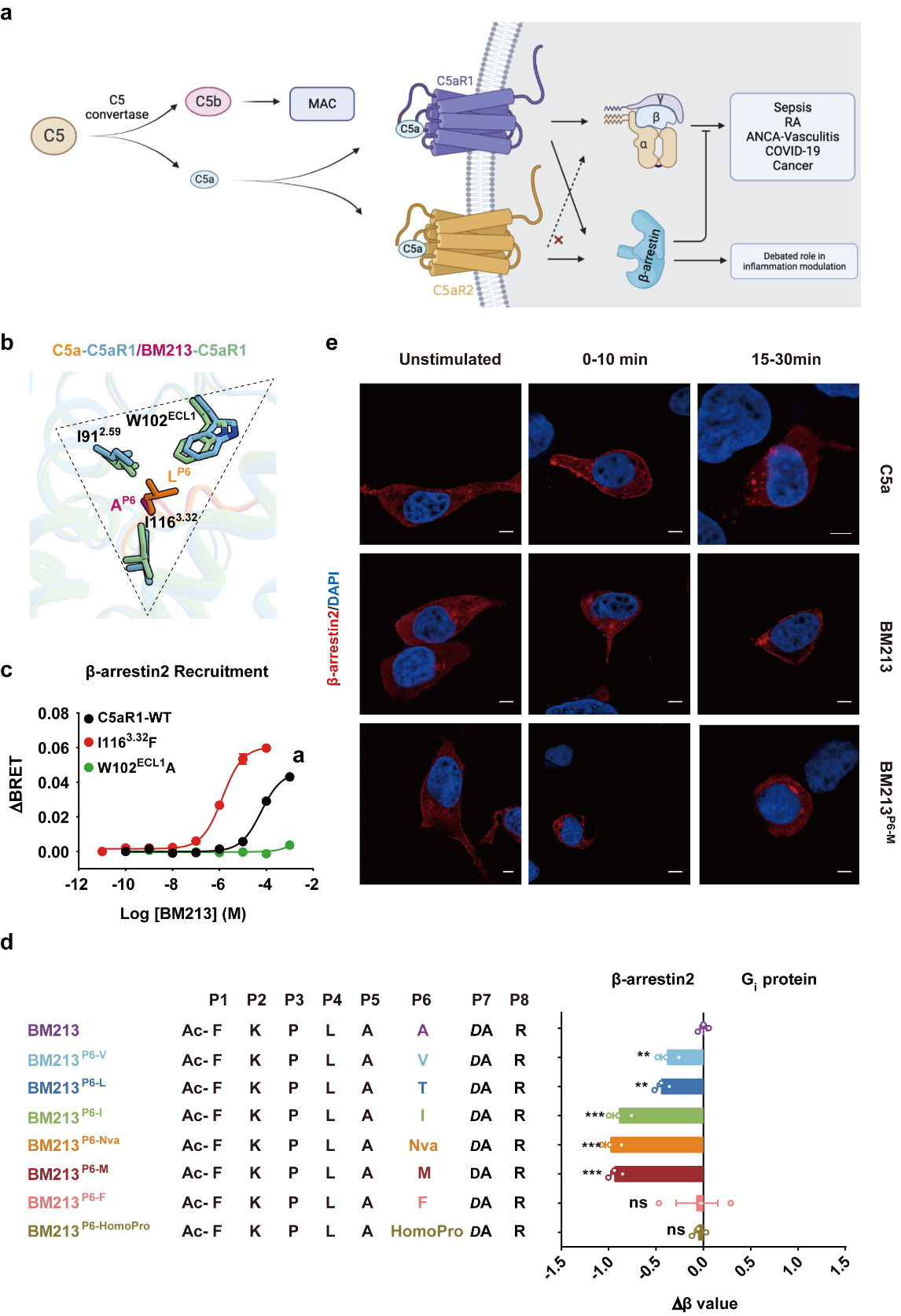
“In the present study, we performed structural and pharmacological analyses of the complement cascade. We solved cryo-electron microscopy (cryo-EM) structures of activated wild-type C5aR1–Gi protein bound to each of C5a, the hexapeptidic agonist C5apep (which has functional bias in terms of trafficking and cellular outcomes17), and the G protein-biased agonist BM213. We also solved the structure of the C5aR1 (I1163.32A) mutant–Gi protein complex bound to the ligand C089. The structures revealed a three-site binding mode of C5a to C5aR1 and a mechanism for diverse ligand recognition. Together with functional assay results, our data elucidate the signaling features of β-arrestin-biased ligands and identifies a “zipper-like” hydrophobic interface required for conformational changes in the receptor.” (Introduction)
Yuying Feng, a postdoctoral fellow of the West China Hospital, Chang Zhao, a doctoral student, Yue Deng, a research assistant, Heli Wang, a master program student, and Liang Ma, an associate research fellow, are the co-first authors of the thesis. Zhenhua Shao, Ping Fu, and Wei Yan are the co-corresponding authors.This work was supported by the National Natural Science Foundation of China (32100988 to W.Y., 31972916 to Z.S.), Science and Technology Department of Sichuan Province (2022ZYD0085 to Z.S., 2021ZYD0080 to W.Y., 2020YFQ0055 to P.F.), Ministry of Technology Department of China grant (2019YFA0508800), 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYYC20023 to Z.S., ZYGD18027 to P.F.) and so forth.
“The complement system plays an important role in the innate immune response to invading pathogens. The complement fragment C5a is one of its important effector components and exerts diverse physiological functions through activation of the C5a receptor 1 (C5aR1) and associated downstream G protein and β-arrestin signaling pathways. --- Here, we present cryo-electron microscopy structures of the activated wild-type C5aR1–Gi protein complex bound to each of the following: C5a, the hexapeptidic agonist C5apep, and the G protein-biased agonist BM213. The structures reveal the landscape of the C5a–C5aR1 interaction as well as a common motif for the recognition of diverse orthosteric ligands. Moreover, combined with mutagenesis studies and cell-based pharmacological assays, we deciphered a framework for biased signaling using different peptide analogs and provided insight into the activation mechanism of C5aR1 by solving the structure of C5aR1I116A mutant–Gi signaling activation complex induced by C089, which exerts antagonism on wild-type C5aR1. In addition, unusual conformational changes in the intracellular end of transmembrane domain 7 and helix 8 upon agonist binding suggest a differential signal transduction process----” (Abstract)
C5aR1β-arrestin biased agonist modification and phenotypic validation
To sum up, “our study provides mechanistic understanding into the ligand recognition, biased signaling modulation, activation, and Gi protein coupling of C5aR1, which may facilitate the future design of therapeutic agents.”
https://www.nature.com/articles/s41422-023-00779-2
