
Reactivity and selectivity are two important aspects in synthetic chemistry. How to regulate reactivity and selectivity to achieve accurate synthesis is a key problem that synthetic chemists have been exploring. Pyridines and related N-heteroareneswidely exist in medicine, pesticide and material molecules. Direct functionalization ofN-heteroarenescan efficiently realize the synthesis or post modification of important molecules, so it is of great significance.
“--- Site-selective C–H functionalization would provide a direct way of making these medicinally-active products3,4,5. For example, nicotinic acid derivatives could be made by C–H carboxylation, but this remains an elusive transformation6,7,8. Here, we describe the development of an electrochemical strategy for the direct carboxylation of pyridines using CO2. The choice of electrolysis setup gives rise to divergent site selectivity: a divided electrochemical cell leads to C5-carboxylation, whereas an undivided cell promotes C4-carboxylation. The undivided cell reaction is proposed to operate via a paired electrolysis mechanism9,10, wherein both cathodic and anodic events play critical roles in altering the site selectivity. Specifically, anodically-generated iodine preferentially reacts with a key radical anion intermediate in the C4-carboxylation pathway via hydrogen-atom transfer, thus diverting the reaction selectivity via the Curtin-Hammett principle11. The scope of the transformation was expanded to a wide range of N-heteroarenes including bi- and terpyridines, pyrimidines, pyrazines and quinolines.” (Abstract)
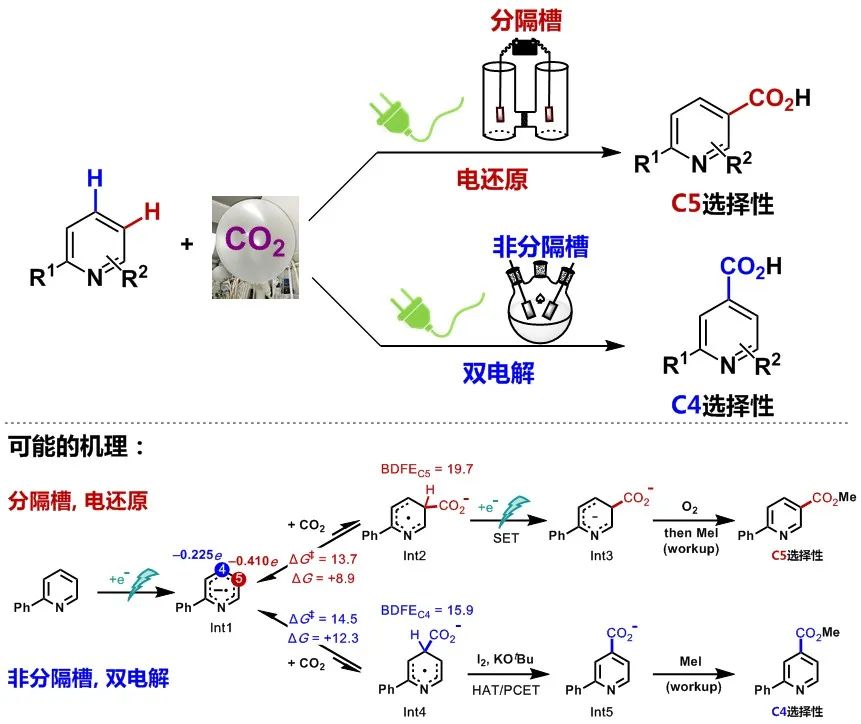
To sum up, this work uses CO2as an ideal carboxyl source to realize the direct and efficient regioselective and controllable carboxylation of azaarenes by changing the electrolytic cell. It has good substrate adaptability and functional group compatibility, which provides a new method for the preparation of important nitrogen-containing heterocyclic carboxylic acid compounds, and also provides a new idea for the resource utilization of CO2, the functionalization of hydrocarbon bonds, and the selective regulation of reactions.
The research findings were published in Nature under the title “Electrochemical Reactor Dictates Site Selectivity of N-heteroarene Carboxylations” (https://www.nature.com/articles/s41586-022-05667-0) (2023) Sichuan University is the first work unit, with Professor Dagang Yu from the College of Chemistry and Professor Song Lin from Cornell University as the co corresponding authors. and Dr. Guoquan Sun from the College of Chemistry of Sichuan University, Dr. Peng Yu and Dr. Wen Zhang from Cornell University as the co first authors. The research was funded by the National Natural Science Foundation of China, the 973 Program of the Ministry of Science and Technology, the Department of Science and Technology of Sichuan Province, Sichuan University and the Beijing National Laboratory for Molecular Sciences.
Links:https://www.nature.com/articles/s41586-022-05667-0
