Prof. Yujiang Fan and research fellow Yong Sun’s research group at the College of Biomedical Engineering/National Engineering Research Center for Biomaterials has published a research paper entitled “An instantly fixable and self-adaptive scaffold for skull regeneration by autologous stem cell recruitment and angiogenesis” in Nature Communications. The co first authors of the research paper are Gonggong Lu, a postdoctoral fellow and Yang Xu, a doctoral student of the College of Biomedical Engineering. The researchers belong to the team of Xingdong Zhang, an academician of CAE.

“Reconstruction of large craniofacial bone defects caused by cerebral trauma still remains highly challenging. Currently, reconstructive operations using autologous/allogeneic grafts suffer from the high cost of bone harvest, limited bone sources, and potential donor site complications. Abundantly available substitutes, including titanium mesh and polyether ether ketone, face drawbacks such as poor stretchability, weak osteointegration, obvious tissue friction, and high Young’s modulus, which tend to constrain intracranial tissue and cannot be implanted immediately after craniectomy. --- Therefore, a functional implant that could be implanted and fixed in defect sites immediately after craniectomy might bring new prospects to adapt to the intracranial microenvironment and mobilize endogenous stem cells (ESCs) for regeneration of cranial tissue.” (Introduction)
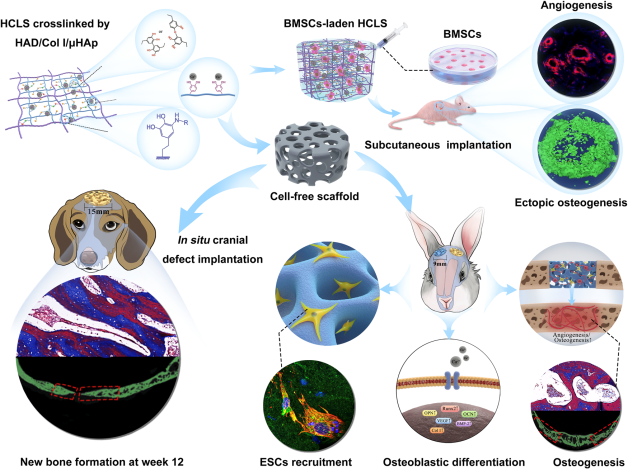
Fig. 1: Clinical treatment of cranial defects by cell-free scaffolds faced significant challenges and imperious demands.
“--- Here, we designed an instantly fixable and self-adaptive scaffold by dopamine-modified hyaluronic acid chelating Ca2+ of the microhydroxyapatite surface and bonding type I collagen to highly simulate the natural bony matrix. It presents a good mechanical match and interface integration by appropriate calcium chelation, and responds to external stress by flexible deformation. Meanwhile, the appropriate matrix microenvironment regulates macrophage M2 polarization and recruits endogenous stem cells. This scaffold promotes the proliferation and osteogenic differentiation of BMSCs in vitro, as well as significant ectopic mineralization and angiogenesis. Transcriptome analysis confirmed the upregulation of relevant genes and signalling pathways was associated with M2 macrophage activation, endogenous stem cell recruitment, angiogenesis and osteogenesis. Together, the scaffold realized 97 and 72% bone cover areas after 12 weeks in cranial defect models of rabbit (Φ = 9 mm) and beagle dog (Φ = 15 mm), respectively.” (Abstract)
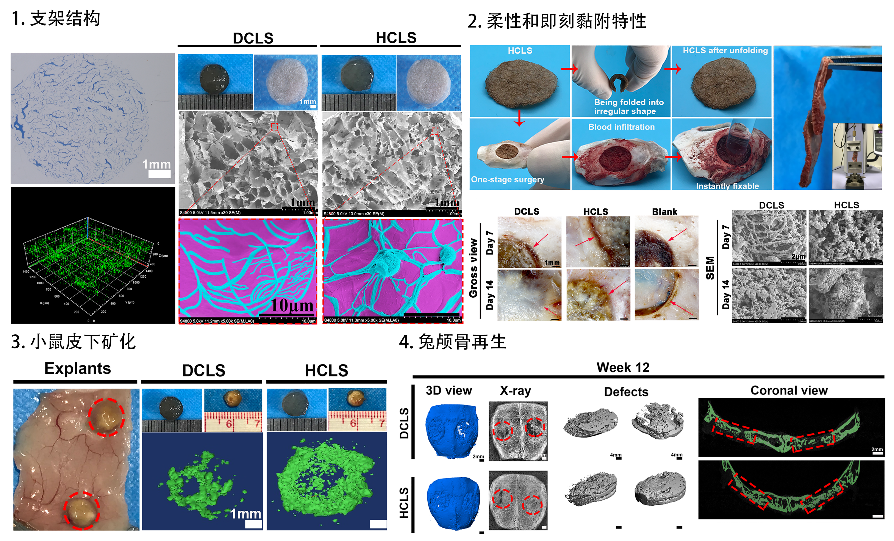
HCLS can enable good mechanical matching and interface integration through tissue adhesion, calcium ion chelation and flexible deformation, leading to skull regeneration.
https://www.nature.com/articles/s41467-022-30243-5
