In collaboration with Manfred Frick’s team of theInstitute of General Physiology, Ulm University, Germany;Burton F. Dickey’s team of the Department of Pulmonary Medicine, University of Texas MD Anderson Cancer Center, and Axel T. Brunger’s team from theDepartment of Molecular and Cellular Physiology, Stanford University, Ying Lai’s team of the State Key Laboratory of Biotherapy and Collaborative Innovation Center of Biotherapy, Sichuan University, published in Nature their latest research results“Inhibition of Ca2+-triggered secretion by hydrocarbon-stapled peptides”on March 23, 2022.
This study revealed the protein targets regulating mucin hypersecretion, developed specific peptide inhibitors, realized the process of inhibiting mucin hypersecretion in asthmatic mouse model for the first time, and successfully prevented the symptoms of airway obstruction in mice.

“Membrane fusion triggered by Ca2+ is orchestrated by a conserved set of proteins to mediate synaptic neurotransmitter release, mucin secretion and other regulated exocytic processes1,2,3,4. For neurotransmitter release, the Ca2+ sensitivity is introduced by interactions between the Ca2+ sensor synaptotagmin and the SNARE complex5, and sequence conservation and functional studies suggest that this mechanism is also conserved for mucin secretion6. --- Here we designed a hydrocarbon-stapled peptide that specifically disrupts Ca2+-triggered membrane fusion by interfering with the so-called primary interface between the neuronal SNARE complex and the Ca2+-binding C2B domain of synaptotagmin-1. In reconstituted systems with these neuronal synaptic proteins or with their airway homologues syntaxin-3, SNAP-23, VAMP8, synaptotagmin-2, along with Munc13-2 and Munc18-2, the stapled peptide strongly suppressed Ca2+-triggered fusion at physiological Ca2+ concentrations. Conjugation of cell-penetrating peptides to the stapled peptide resulted in efficient delivery into cultured human airway epithelial cells and mouse airway epithelium, where it markedly and specifically reduced stimulated mucin secretion in both systems, and substantially attenuated mucus occlusion of mouse airways. Taken together, peptides that disrupt Ca2+-triggered membrane fusion may enable the therapeutic modulation of mucin secretory pathways.”(Abstract)
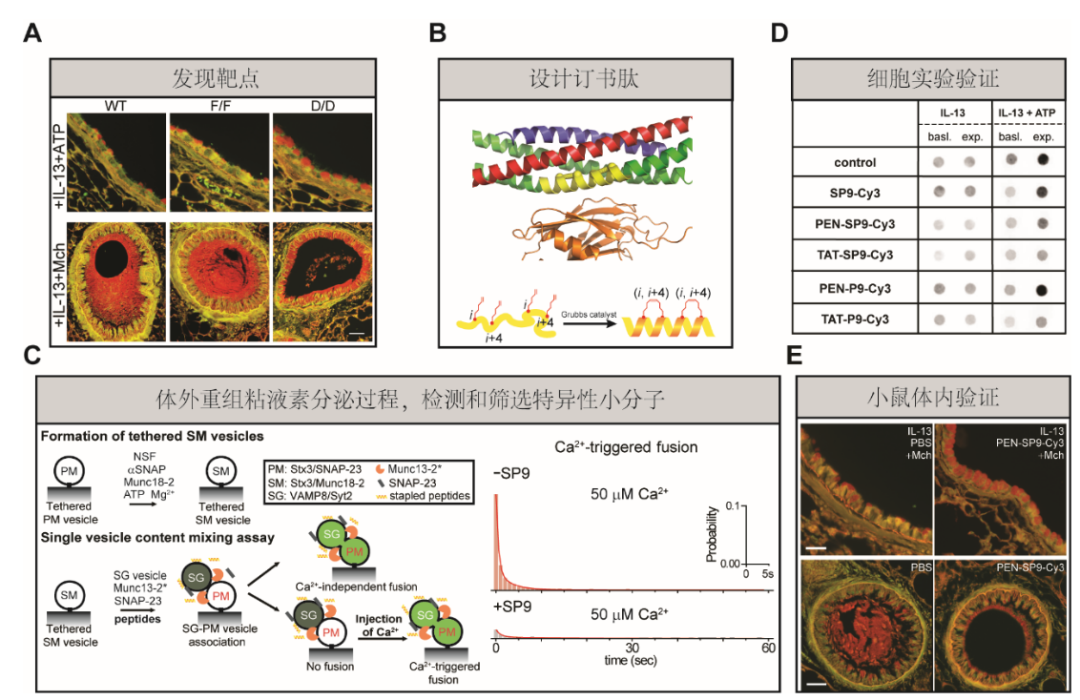
Fig. 1: Mucin secretion defects in Syt2-mutant mice
Being the first and co corresponding author of this study, Ying Lai joined theState Key Laboratory of Biotherapy and Collaborative Innovation Center of Biotherapyin 2019. So far, he has published 31 articles in Nature,Neuron,PNAS, with an H-index of 22 and a total of more than 2,100 citations. The research directions of Lai Ying's research group include: 1. Research on the molecular mechanism of SNARE protein mediated vesicle transport and development of small molecule drugs; 2. Molecular mechanism research and drug development of spike protein mediated virus invasion into host cells; 3. Technology development related to biofilm fusion. The research group recruits postdoctoral and scientific research assistants all the time.
Research team website:http://sklb.scu.edu.cn/detail.html?type=postgraduateDetail&id=342
Article link:https://doi.org/10.1038/s41586-022-04543-1
