On August 11, 2021, Zhaoming Su's team of the State Key Laboratory of Biotherapy(SKLB), West China Hospital, Sichuan University, Kaiming Zhang's team of the University of Science and Technology of China, and Wah Chiu and Rhiju Das's team of Stanford University jointly published in Nature an online research paper entitled "Cryo-EM Structures of Full-length Tetrahymena Ribozyme at 3.1 Å Resolution".
RNA has important biological functions. Many noncoding RNAs can be folded into complex three-dimensional structures to exercise or participate in important life processes, such as catalysis, transcription and translation regulation. However, due to the lack of RNA three-dimensional structure information, the current understanding of the relationship between RNA three-dimensional structure and function is still limited. Cryoelectron microscopy has become an indispensable technology for the structural analysis of biological macromolecules. Again, due to the strong heterogeneity of RNA structure, the acquisition of high-resolution Cryo-EM structure of pure RNA still remains a great challenge. Previously, there were less than 10 pure RNA structures with a resolution better than 5 Å in the Cryo-Em database, and the highest resolution was 3.7 Å. In this study, the researchers analyzed thestructures of the full-length Tetrahymenaribozyme in substrate-free and bound states at a resolution of 3.1 Å.

Fig. 1: Cryo-EM reconstruction of apo L-21 scal ribozyme.
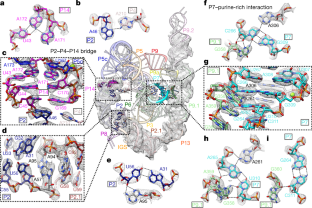
Fig. 2: Structural insights in the peripheral regions.
“Single-particle cryogenic electron microscopy (cryo-EM) has become a standard technique for determining protein structures at atomic resolution1,2,3. ---Here we report cryo-EM structures of the full-length Tetrahymenaribozyme in substrate-free and bound states at a resolution of 3.1 Å. Newly resolved peripheral regions form two coaxially stacked helices; these are interconnected by two kissing loop pseudoknots that wrap around the catalytic core and include two previously unforeseen (to our knowledge) tertiary interactions. The global architecture is nearly identical in both states; only the internal guide sequence and guanosine binding site undergo a large conformational change and a localized shift, respectively, upon binding of RNA substrates. These results provide a long-sought structural view of a paradigmatic RNA enzyme and signal a new era for the cryo-EM-based study of structure–function relationships in ribozymes.” (Abstract)
The State Key Laboratory of Biotherapy, West China Hospital, Sichuan University, the Department of Geriatrics and National Clinical Research Center for Geriatrics,and the National Clinical Medical Research Center for Geriatric Diseases are the first work units of this study. Zhaoming Su is the first author and co-corresponding author of this paper, and Kaiming Zhang and Dr. Kalli Kappel of Stanford University are co first authors. This work was supported by the National Science Foundation (DGE-114747 to K.K. and DGE-1656518 to R.R.); Gabilan Stanford Graduate Fellowship to K.K.; Sichuan University start-up funding 20822041D4057 and Natural Science Foundation of China (NSFC82041016 and 32070049 to Z.S) and so on.Moreover,this study received great support from Yuquan Wei, an academician of the Chinese Academy of Sciences.
