Research fellow Haohao Dong’s research team from the State KeyLaboratory of Biotherapy and Cancer Center(SKLBCC), West China Hospital has recently published a research paper entitled“Structural Basis for Bacterial Lipoprotein Relocation by the Transporter LolCDE”in Nature Structural & Molecular Biology.
The research work was completed by Haohao Dong’s research team, the team of Prof. Xing Zhang, director of the Center of Cryo Electron Microscopy, Zhejiang Universityand the team of Prof. Changjiang Dong, Biomedical Research Centre, Norwich Medical School, University of East Anglia, UK. The first authors are Dr. Xiaodi Tang, associate professor of SKLBCC; Dr. Shenghai Chang, director of Center of Cryo Electron Microscopy, Zhejiang University; Ke Zhang and Qinghua Luo of SKLBCC. The research received strong support from Prof. Xiawei Wei and Prof. Xiaofeng Zhu of Sichuan University and Zhengyu Zhang, a research fellow of Wuhan University.

In this study, we analyzed the protein machine LolCDE (apo), which is used to transport outer membrane lipoproteins in multidrug-resistant gram-negative bacteria, and its six high-resolution frozen electron microscopic structures combined with substrate lipoproteins, ADP, ATP isomorphic inhibitor AMP-PNP and periplasmic protein Lola. We also showed four intermediate conformations in its transport function.
“Lipoproteins in the outer membrane of Gram-negative bacteria are involved in various vital physiological activities, including multidrug resistance. Synthesized in the cytoplasm and matured in the inner membrane, lipoproteins must be transported to the outer membrane through the Lol pathway mediated by the ATP-binding cassette transporter LolCDE in the inner membrane via an unknown mechanism. Here, we report cryo-EM structures of Escherichia coli LolCDE in apo, lipoprotein-bound, LolA-bound, ADP-bound and AMP-PNP-bound states at a resolution of 3.2–3.8 Å, covering the complete lipoprotein transport cycle. Mutagenesis and in vivo viability assays verify features of the structures and reveal functional residues and structural characteristics of LolCDE. The results provide insights into the mechanisms of sorting and transport of outer-membrane lipoproteins and may guide the development of novel therapies against multidrug-resistant Gram-negative bacteria.”(Abstract)
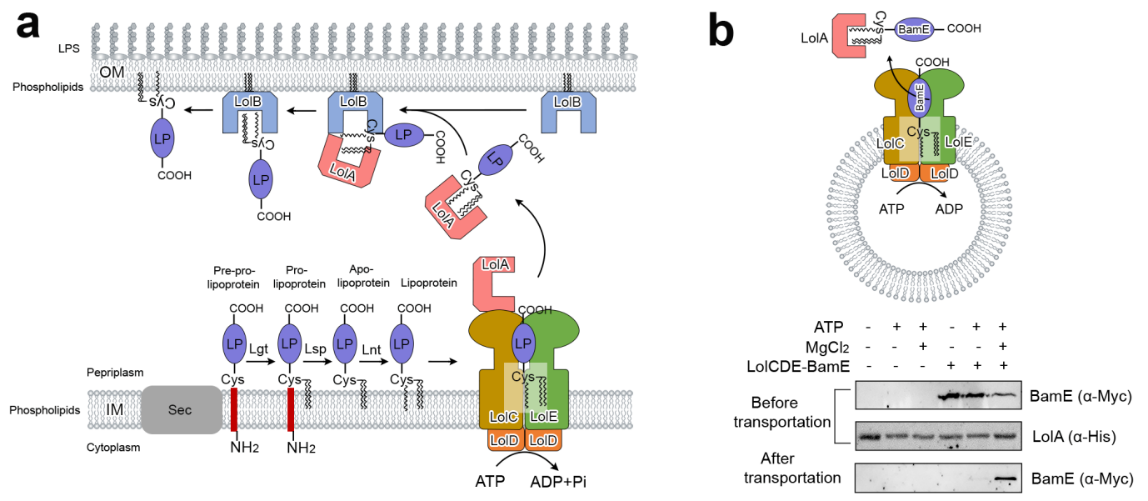
In conclusion, this study revealed the molecular details of the binding between the protein machine LolCDE and the substrate lipoproteins, and carried out site directed single mutation and in vitro construction of protein phospholipid vesicles. This study is of great significance for understanding the mechanism of action of other type VII ABC transporters and promoting drug development for the treatment of multidrug-resistant gram-negative bacteria.
Article link:https://dx.doi.org/10.1038/s41594-021-00573-x
