Professor Dawen Niu's team from the School of Chemical Engineering has enabled the efficient, asymmetric O-propargylation of secondary aliphatic alcoholsfor the first time. The results were published in an online research paper entitled "Asymmetric O-propargylation of Secondary Aliphatic Alcohols" in Nature Catalysis on June 1, 2020. The team conducted the whole research at Sichuan University. Professor Dawen Niu is the corresponding author and Sichuan University is the first work unit. Renzhe Li, an SCU doctoral student is the first author of this paper, and Daqi Liu, a master program student is the second author.

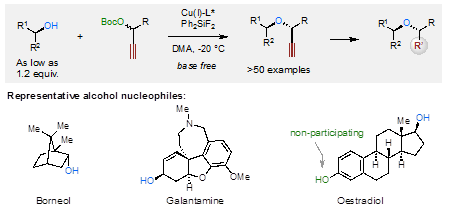
“The asymmetric O-alkylation of secondary aliphatic alcohols remains a substantial challenge in chemistry. Such a challenge largely stems from the steric demand of each reactant, in addition to the relatively low nucleophilicity of alcohols. Here, we report the development of a base-free, Cu-catalysed propargylic substitution reaction that enables the efficient, asymmetric O-propargylation of secondary aliphatic alcohols. Mechanistic studies implied key factors to slow down the undesired decomposition process of electrophiles in this reaction, which opened up the possibility of using secondary aliphatic alcohols as nucleophilic substrates. This asymmetric O-alkylation reaction proceeds under almost neutral conditions, tolerates a broad scope of functional groups and shows remarkable chemoselectivities. This method is amenable to the modification of natural products and commercial drugs. The products obtained could be readily elaborated to various classes of enantioenriched α,α′-disubstituted ethers that are difficult to access by other methods.” (Abstract) Speaking of the research, Prof. Niu said:“ I was moved by the indomitable and persistent scientific research spirit that my students have exhibited during this project.”
This research work is supported by funding from the National Key Research and Development Program (2018YFA0903300) and National Natural Science Foundation of China (21922106 and 21772125), and start-up funding from Sichuan University and The Open Project of the State Key Laboratory of Natural Medicines (3144060211).
Article link: https://www.nature.com/articles/s41929-020-0462-9
