Ying Xia’s research team from the Public Health and Preventive Medicine Experimental Center of West China Fourth Hospital , recently published a research paper entitled "Directing Group-Free Formal Suzuki − Miyaura Coupling of Simple Ketones Enabled by Activation of Unstrained C − C Bonds" online in Angew. Chem. Int. Ed. The first author of the paper is Jiangkun Huang, a Class 2020 doctoral student of Sichuan University, and Prof. Ying Xia is the sole corresponding author of the paper. The first work unit of the paper is West China School of Public Health (West China Fourth Hospital) of Sichuan University/State Key Laboratory of Biotherapy.
The Suzuki − Miyaura coupling (SMC) reaction has been greatly developed since it was reported in 1979. The classic SMC reaction takes C − X of aryl halides as the electrophilic coupling sites. In the following decades, C − O, C − N, C − S and other carbon heterobonds as the electrophilic coupling sites have also been gradually developed.
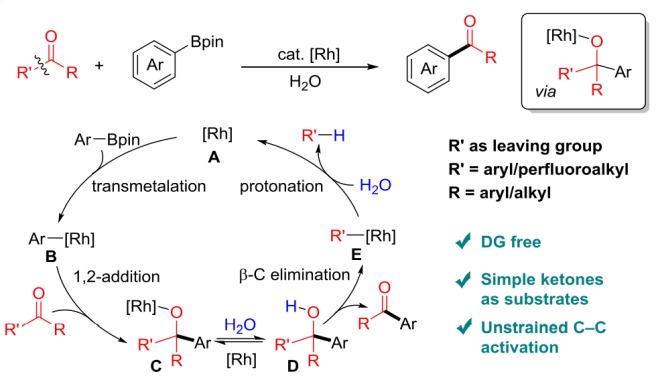
“The use of ketones as electrophiles to couple with arylboronic acid derivatives via C−C bond activation has become a significant progress in the area of Suzuki–Miyaura coupling (SMC) reaction, in which a permanent or temporary directing group is often required to promote the activation of the unstrained C−C bond via oxidative addition. Herein, we disclosed the first example of directing group free formal SMC reaction of simple ketones with arylboronates via Rh-catalyzed unstrained C−C bond activation. A wide range of simple ketones, including aryl alkyl ketones, diaryl ketones and aryl perfluoroalkyl ketones, can serve as electrophiles to participate in the SMC reaction with aryl or perfluoroalkyl as the leaving group. The key to the success of this reaction is by means of nucleophilic addition/β-carbon elimination sequence that can activate the unstrained ketone carbonyl C−C bond without the assistance of directing group.” (Abstract)
Ying Xia is a scholar of the National Talent Plan and Sichuan Talent Plan. She was introduced to SCU by the West China School of Public Health/West China Fourth Hospital and the State Key Laboratory of Biotherapy in May 2019. At present, she is the director and doctoral students adviser of the Public Health and Preventive Medicine Experimental Center of the West China School of Public Health/West China Fourth Hospital. So far, She has published more than 60 SCI papers in the top or important journals in the field, including more than 40 papers in her capacity of a first or corresponding author, with a total number of citations of more than 4,000.
The work was funded bythe National Natural Science Foundation of China, Key Research and Development Program of Sichuan Province and start-up funding from Sichuan University.
https://onlinelibrary.wiley.com/doi/10.1002/anie.202211080
