The Cell PressjournalTrends in Biochemical Sciences (IF 15.678) has recently published a review entitled “Targeting Metabolic-Redox Circuits for Cancer Therapy” by the team of Professor Canhua Huang (a Changjiang Scholars Program endowed professor; and chief scientist for National 973 Program; PI, State Key Laboratory of Biotherapy, West China Hospital, Sichuan University). Dr. Kui Wang (an associate research fellow) and Dr. Jingwen Jiang are the first authors, and Professor Canhua Huang is the corresponding author. In this review, they discuss how cancer cell metabolism and redox signaling are intertwined.

Metabolic reprogramming and oxidative stress are two major characteristics of cancer. Unlike their normal counterparts which employ the tricarboxylic acid (TCA) cycle and oxidative phosphorylation (OXPHOS) for ATP generation, cancer cells exhibit an accelerated rate of glycolysis in the presence of oxygen (aerobic glycolysis) to fulfill the high biosynthetic and bioenergetic demands for rapid and sustained proliferation. Metabolic abnormalities contribute to the accumulation of reactive oxygen species (ROS) in cancer cells. Moderate levels of ROS can serve as signaling molecules to regulate pro-survival pathways, whereas high levels of ROS can lead to biomolecular damage and even cell demise. Therefore, cancer cells have developed powerful non-enzymatic and enzymatic ROS-scavenging systems to cope with oxidative damage for cell survival.
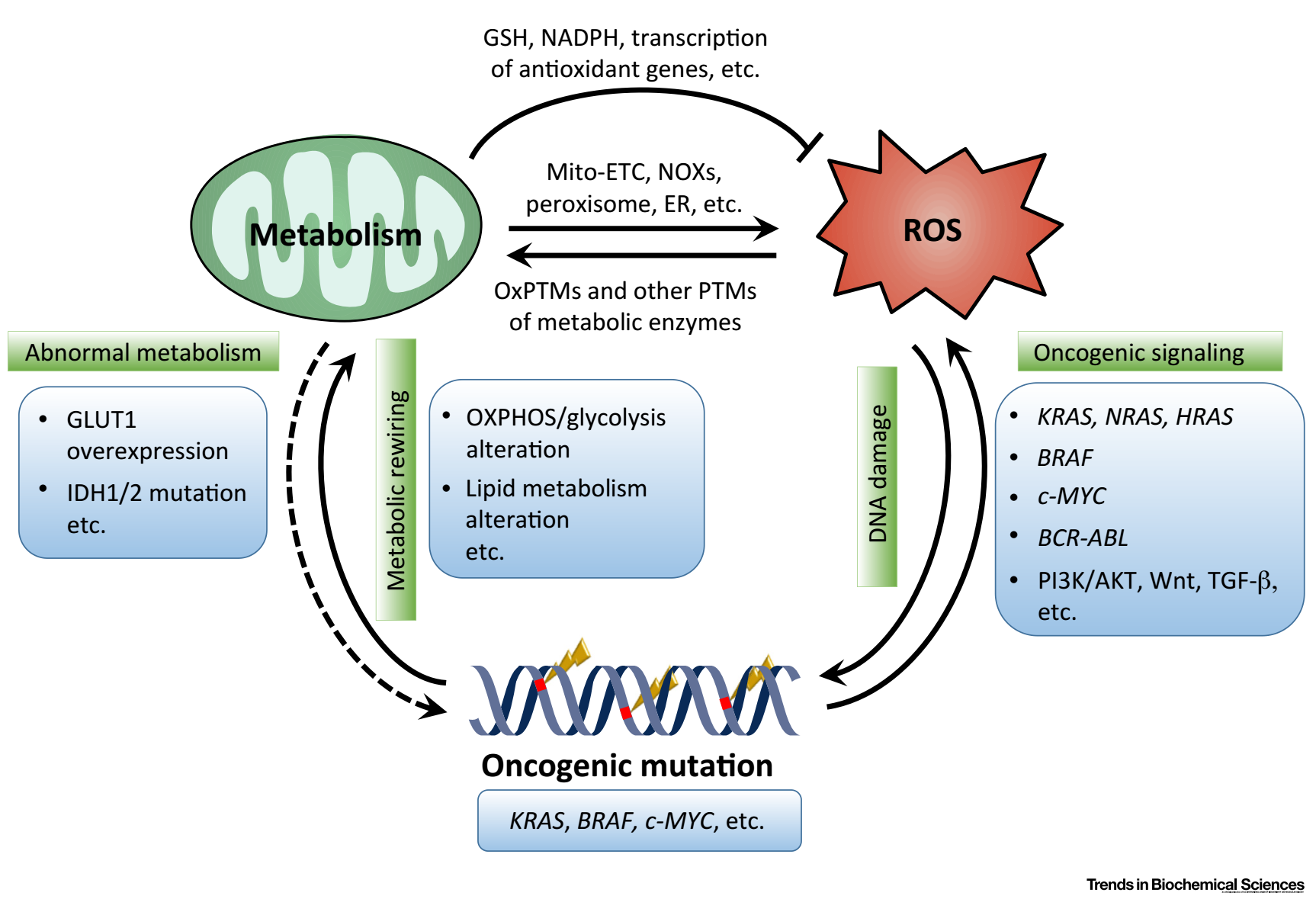
Cancer cell metabolism and redox signaling are intricately intertwined to constitute complex metabolic-redox circuits, which comprise the following critical lines: Metabolic alterations in cancer cells induce ROS production via Mito-ETC, NOXs, peroxisomal β-oxidation, or ER stress; in response to ROS accumulation, cancer cells rewire metabolic pathways to activate intrinsic antioxidant systems (such as NADPH and GSH, etc.) to prevent oxidative damage. Specifically, ROS can directly (via oxPTMs) or indirectly (via other PTMs) alter the function of metabolic enzymes, enabling the evolvement of reductant-producing metabolic patterns; Sustained moderate oxidative stress can induce genomic instability and DNA damage, and may result in oncogenic mutations (includingKRAS,BRAF,c-MYC, etc.). Oncogenic mutations in turn advance tumorigenesis and tumor progression, at least partially, through altering metabolic phenotypes or promoting ROS production. Aberrant metabolism or ROS accumulation in cancer cells is capable of inducing further oncogenic mutations that benefit tumor cell growth and proliferation.
The dynamic metabolic-redox circuits contribute to sustained growth of cancer cells and confer therapeutic resistance. A thorough understanding of the metabolic-redox circuits of cancer cells may help overcome the metabolic or redox adaptations in response to cancer therapy and open up new therapeutic windows for future cancer intervention.
Professor Huang’s team has long been focused on investigating the roles of redox signaling and metabolism in tumorigenesis and progression. The research work has been funded by the National Natural Science Foundation of China and the National 973 Basic Research Program of China.
Full article link:https://doi.org/10.1016/j.tibs.2019.01.001
