On May 4, the British Medical Journal (BMJ), one of the four major journals of clinical medicine, publisheda clinical practice guideline for PCSK9 inhibitors and ezetimibefor the reduction of cardiovascular events as its cover article, “PCSK9 inhibitors and ezetimibe for the reduction of cardiovascular events: with risk-stratified recommendations.”
Quikui Hao, associate professor of the Center of Gerontology and Geriatrics /National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University, is the first author. Sheyu Li, endocrinologist of the Department of Endocrinology and Metabolism, West China Hospital, and Dr.Nicolas Delvaux,Department of Public Health and Primary Care and MAGIC Primary Care, KU Leuven, Belgium, are the corresponding authors. The West China Hospital is the leading work unit.This is the first international evidence-based clinical practice guideline for cholesterol lowering drugs that has been completed with mainland Chinese scholars as the chief scientists.

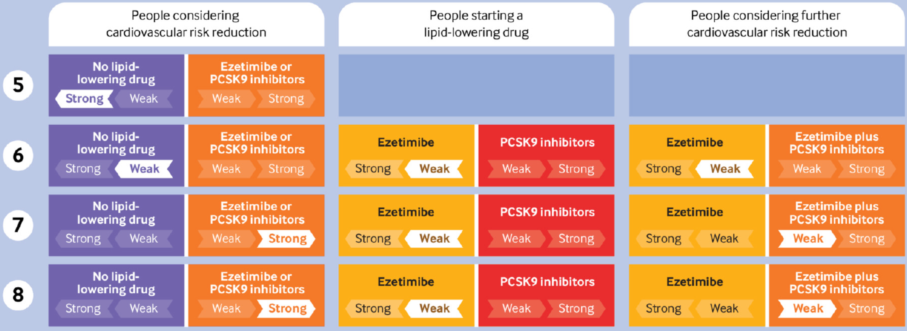
Being drafted for adult cardiovascular disease prevention, the guideline is to assist general practitioners, cardiovascular doctors, endocrinologists and metabolic doctors, physicians and geriatricians engaged in relevant clinical work all over the world.The guideline panel provided mostly weak recommendations as follows:
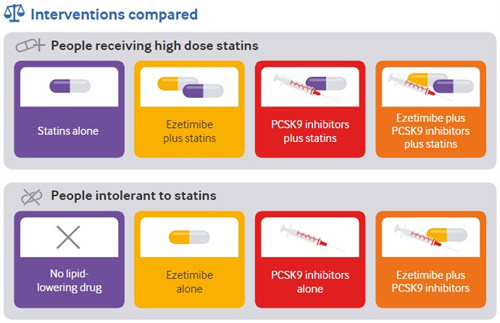
•For adults taking high dose statins, with LDL cholesterol >70 mg/dL (1.8 mmol/L) certainty).
To whom do they apply?
The recommendations apply to adults with LDL cholesterol >70 mg/dL (1.8mmol/L) considering further reduction in risk of CV events who are already taking statins or are intolerant to statins.
Please note that people who previously reported severe muscle symptoms when taking statins (may be labelled as intolerant to statins) should first consider restarting statins at a low dose to reduce their cardiovascular risk, as many could have a nocebo effect or combined effect.
This guideline represents a shift from the traditional focus on lipid level goals to a focus on reducing an individual’s overall cardiovascular risk. Clinicians need to identify patients’ individual cardiovascular risks to apply these risk-stratified recommendations. The use of these recommendations therefore warrants explicit judgments of individual baseline cardiovascular risk, using credible risk calculators applicable to specific geographic regions. Most risk prediction tools use a cardiovascular risk over a period of 10 years, but this is not consistent with many trials on interventions for cardiovascular risk, which rarely extend beyond five years. The panel chose the most widely applicable calculator (PREDICT) to estimate patients’ risk, of mortality, non-fatal myocardial infarction, and non-fatal stroke over five years, in part because PREDICT provides risk estimates for both primary and secondary prevention populations (appendix 2).
Risk stratification approach and baseline risk estimation for the guideline
The panel estimated baseline risks for individual outcomes (mortality, non-fatal myocardial infarction, and non-fatal stroke) over a five year timeframe. We used medians of the risk within each risk category from the PREDICT cohort as the baseline risk estimates.
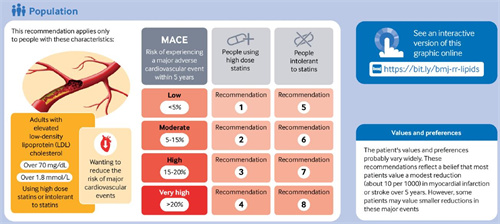
The guideline presentsour approach to risk stratification, with key characteristics to consider displayed in the infographic.
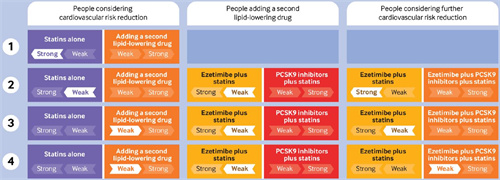
Low risk (<5% five year risk of major adverse cardiovascular event (MACE)): We recommend not adding a second lipid-lowering drug (strong recommendation)
Moderate risk(5-15% five year risk of MACE): We suggest not adding a second lipid-lowering drug; but for those who are considering adding a second lipid-lowering drug, we suggestadding ezetimibe first (weak recommendation); we recommend not adding a PCSK9 inhibitor to ezetimibe (strong recommendation)
High risk (15-20% five year risk of MACE):We suggest adding a second lipid-lowering drug, preferably ezetimibe first; we suggest not adding a PCSK9 inhibitor to ezetimibe (weak recommendation)
Very high risk (>20% five year risk of MACE): We suggest adding a second lipid-lowering drug, preferably ezetimibe first; we suggest adding a PCSK9 inhibitor to ezetimibe (weak recommendation).
For adults intolerant to statins with LDL cholesterol >70 mg/dL (1.8 mmol/L)
‐Low risk (<5% five year risk of MACE): We recommend not using a lipid-lowering drug (strong recommendation)
‐Moderate risk (5-15% five year risk of MACE): We suggest not using a lipid-lowering drug; but for those who are considering using a lipid-lowering drug, we suggest adding ezetimibe first (weak recommendation); we recommend not adding a PCSK9 inhibitor to ezetimibe (strong recommendation)
‐High risk (15-20% five year risk of MACE) and very high risk (>20% five year risk of MACE): We recommend using a lipid-lowering drug (strong recommendation), preferably ezetimibe first; we suggest adding a PCSK9 inhibitor to ezetimibe (weak recommendation).
The guideline is based on the high-quality evidence synthesis study of Dr. Safi Khan of Houston Methodist DeBakey heart and vascular center (BMJ 2022; 377: e069116, DOI: 10.1136 / bmj-2021-069116), which gives detailed and quantitative cardiovascular benefits of ezetimibe and PCSK9 inhibitors. Meanwhile, two safety evidence synthesis studies led by Sheyu Li (BMJ Med 2022; 1, DOI: 10.1136/bmjmed-2022-000134; heart 2022;
doi: 10.1136/heartjnl-2021-320556) made quantitative analysis on the adverse reactions of two cholesterol lowering drugs respectively.
This guideline was funded by 1·3·5 project for disciplines of excellence–Clinical Research Incubation Project, West China Hospital, Sichuan University (Nos. 19HXFH011, ZYGD18022 and 2020HXF011).
An international panel including patients, clinicians, and methodologists from China, Canada, Belgium, Norway, the U.S.A, Switzerland, New Zealand, Saudi Arabia, Australia, Holland, Cameroon, the UK and Romania produced these recommendations following standards for trustworthy guidelines and using the GRADE approach.
This guideline is not only the first evidence-based clinical practice guideline for cholesterol lowering drugs, which is led by mainland Chinese scholars, but also another landmark achievement of Cochrane China Center/MAGIC China Center, which is located at West China Hospital of Sichuan University, after the internationalclinical practice guideline fordiabetes II in 2021.
https://magicevidence.org/match-it/220504dist-lipid-lowering-drugs/
https://doi.org/10.1136/bmj-2021-069066
