“SARS-CoV-2 contains a single-stranded positive-sense RNA genome that encodes 16 non-structural proteins (NSPs), several accessory proteins, and four essential structural proteins, including small envelope (E) protein, nucleocapsid (N) protein, spike (S) glycoprotein, and matrix (M) protein6. The interaction between the virus and its host is central to completing the virus lifecycle, including virus entry, replication, and dissemination. Specifically, angiotensin-converting enzyme 2 (ACE2) and transmembrane serine protease 2 (TMPRSS2)7have been regarded as two essential host determinants for SARS-CoV-2 infection and pathogenesis. When SARS-CoV-2 invades, the S glycoprotein initially engages ACE2 as a cellular receptor for entry and then is enzymatically activated by TMPRSS2, which is essential for efficient fusion and release of the virus contents into the host cell cytosol.”(introduction)
The research team of Xianghui Fu at theDivision of Endocrinology and Metabolism, State Key Laboratory of Biotherapy and Cancer Center, West China Hospital, Sichuan University has published in Nature Communications the research results entitled "RNA G-quadruplex in TMPRSS2 reductions Sars-CoV-2 infection".

RG4 is a non-classical RNA high-level structure composed of nucleotide chains rich in tandem repeat guanine bases (g); It affects gene expression by regulating RNA cutting, transport and translation, and participates in various life activities and the occurrence and development of a variety of diseases.
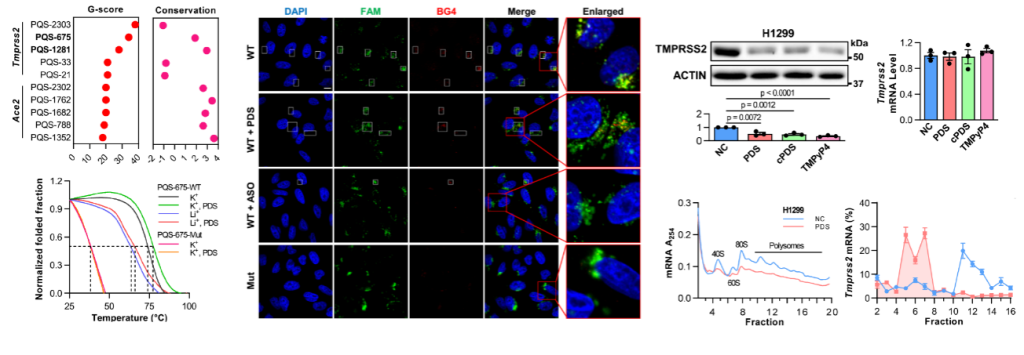
Fig.1. RG4 inhibits TMPRSS2 translation.
“Herein, we identify the role of RG4 in SARS-CoV-2 infection and pathogenesis. The presence of RG4 in both SARS-CoV-2 genome and host factors are characterized by a combination of multiple bioinformatics, biochemical, and biophysical assays. The biological and pathological importance of RG4 in SARS-CoV-2 pathogenesis is then exemplified by a single RG4 in Tmprss2. This canonical 3-quartet RG4 structure in the open reading frame (ORF) region can inhibit Tmprss2 translation. Correspondingly, G-quadruplex (G4)-specific stabilizers attenuate SARS-CoV-2 entry to host cells in both pseudovirus cell systems and mouse models.”(introduction)
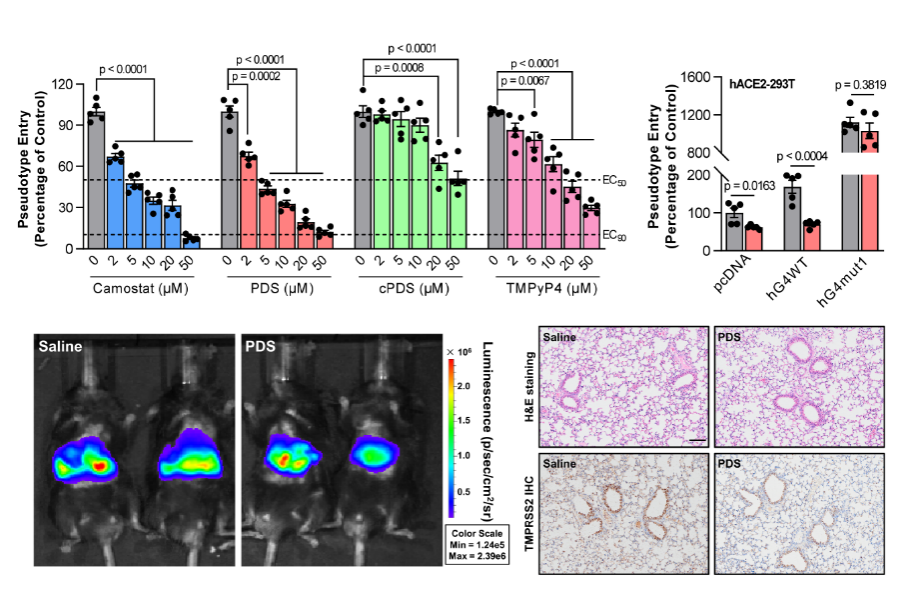
Fig.2. RG4 hinders the efficacy of SARS-CoV-2 infection in vivo.
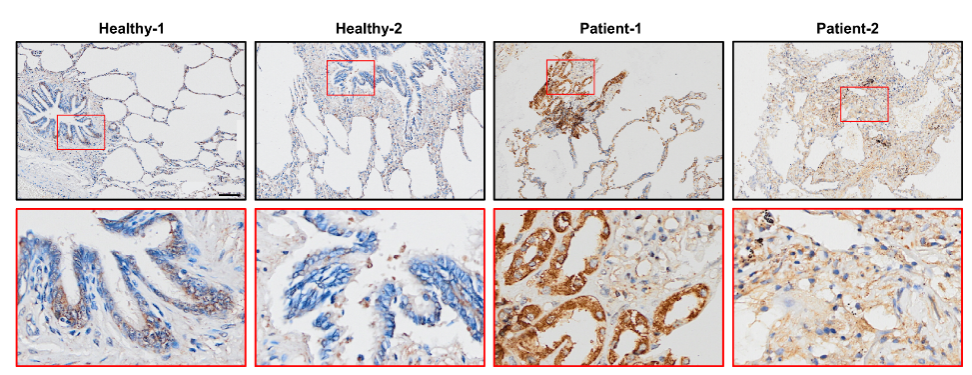
Fig.3. The protein level of TMPRSS2 was increased in the lung tissue of covid-19 patients.
All in all, these findings uncover a mechanism underlying SARS-CoV-2 infection and suggest the potential of RG4-targeting strategies for COVID-19 prevention(Fig.4). They provide a new idea for the discovery of COVID-19 targets.
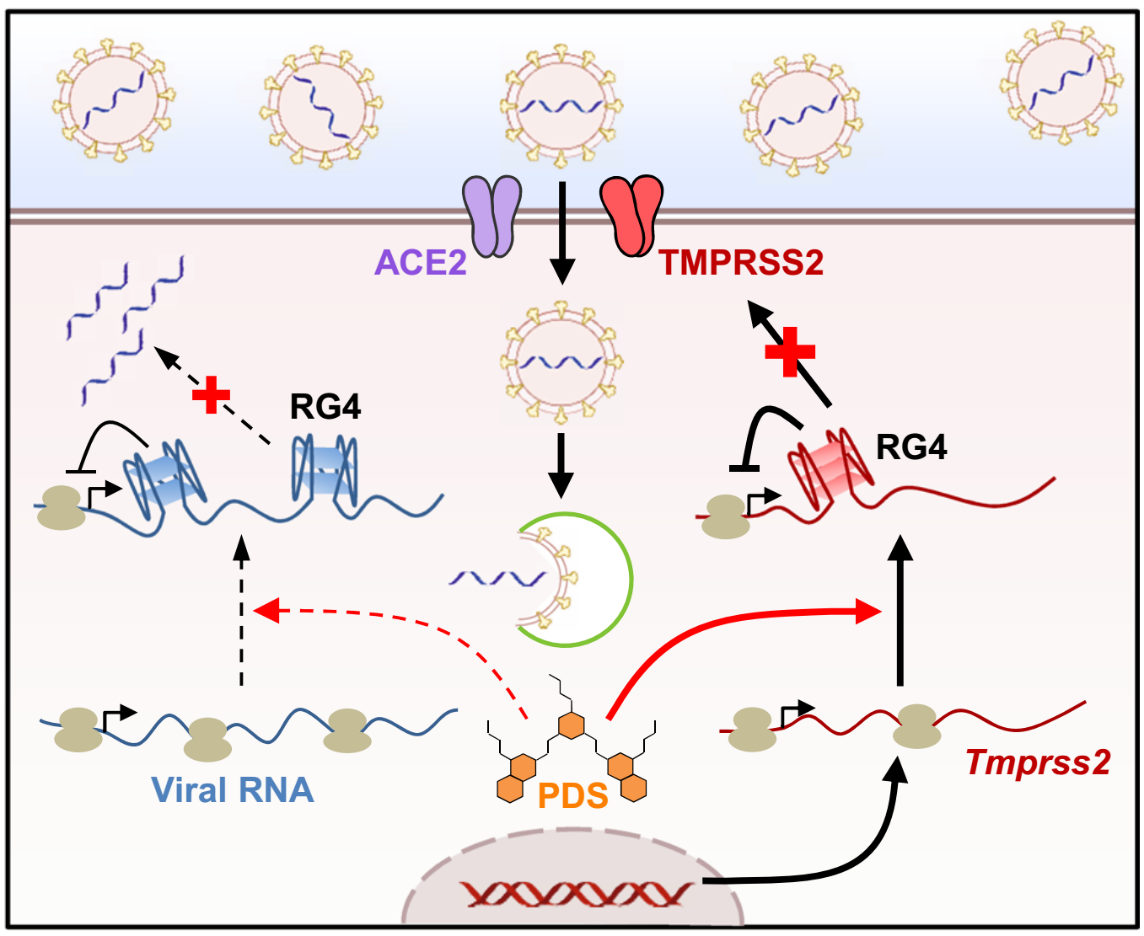
Fig.4. RG4 in murine Tmprss2 mRNA inhibits its protein expression.
“Combining bioinformatics, biochemical and biophysical assays, we demonstrate the presence of RG4s in both SARS-CoV-2 genome and host factors. The biological and pathological importance of these RG4s is then exemplified by a canonical 3-quartet RG4 within Tmprss2, which can inhibit Tmprss2 translation and prevent SARS-CoV-2 entry. Intriguingly, G-quadruplex (G4)-specific stabilizers attenuate SARS-CoV-2 infection in pseudovirus cell systems and mouse models. Consistently, the protein level of TMPRSS2 is increased in lungs of COVID-19 patients. Our findings reveal a previously unknown mechanism underlying SARS-CoV-2 infection and suggest RG4 as a potential target for COVID-19 prevention and treatment.”(Abstract)
https://www.nature.com/articles/s41467-022-29135-5
