Recently, a team led by Professor Dawen Niu, awardee of National High Level Program funding, made progress in the synthesis of carbohydrate derivatives. The results were published as a communication inJ. Am. Chem. Soc., a top multidisciplinary journal covering fields of chemistry, material science, and biology. Liang Gong and Hong-Bao Sun were co-first authors of this paper, and Professor Dawen Niu the corresponding author.

Carbohydrates play important roles in essentially all physiological and pathological events, including immunization, fertilization, and the development and metastasis of cancer.Research on carbohydrates is therefore a significant branch of chemistry, biology, life science, and material science. Exploration of the functions of carbohydrates has proven fruitful platform to understand key biological processes and develop novel pharmaceuticals. However, the procurement, modification, and structural identification of carbohydrates represent a huge, long-standing challenge, due to their structural complexity. Lack of efficient methods to prepare carbohydrates and their derivatives to a large extent hamper the study of carbohydrates in many fields.
Professor Dawen Niu’s group has been focusing on the development of novel methods of preparing carbohydrate derivatives, and made important progresses in recent years. ( For representative publications, see: Chem, 2017,3, 834; Angew. Chem. Int. Ed., 2017,56, 7213; Angew. Chem. Int. Ed., 2018, 57, 320; Angew. Chem. Int. Ed., 2018,57, 9456; Carbohydr. Res.2019, 474, 16-33 ). Lately, this group reported breakthrough in the synthesis of C-aryl glycals. In this project, they have established the use of 1-sulfonyl glycal as a novel reagent for the venerable Suzuki-Miyaura cross-coupling reaction, to make variousC-aryl glycals ( Figure 1 ). They found that the readily available and bench-stable 1-sulfonyl glycals could be used as surrogates of 1-iodo glycals, the latter of which are difficult to access and inherently unstable. These reactions proceeded under very mild conditions, and displayed remarkable functional group tolerance, thereby allowing synthesis of many previously inaccessibleC-aryl glycals. Through careful mechanistic studies, the group proposed the adjacent O-atom plays critical role in effecting this transformation.
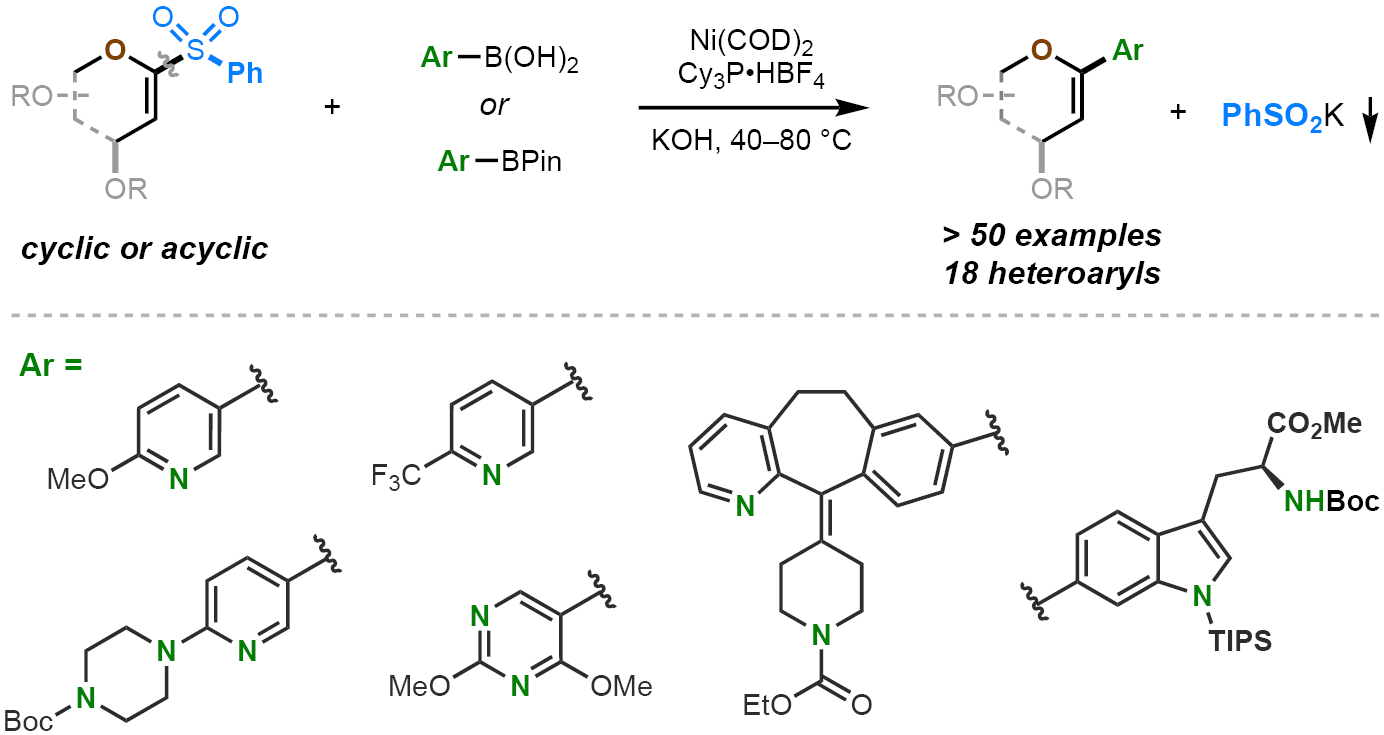
Figure 1. The Use of 1-Sulfonyl Glycals in the Synthesis ofC-Aryl Glycals
This method could be applied in the synthesis of SGLT2 inhibitors such as ipragliflozin, an anti-diabetic drug. Combining this method with C-H borylation reaction, the authors realized glycosylation of trypthophan. Moreover, this method has enabled a more straightforward synthesis of some potent human NK1 antagonists ( Figure 2 ). The operational ease, the functional group tolerance, the ready availability of starting materials bode well for the broad application of this methods in carbohydrate chemistry and medicinal chemistry.
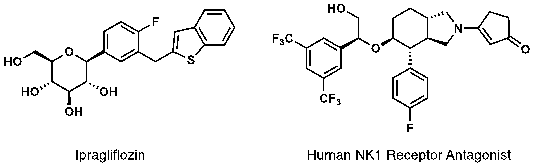
Figure 2. Drugs and Drug Candidates Made by This Method
Original link: https://pubs.acs.org.ccindex.cn/doi/10.1021/jacs.9b02312
