Under the leadership of Sichuan University ( SCU ) Distinguished Professor Ben-He Zhong, Professor Xiao-Dong Guo’s research team at the Phosphorus-based Functional Materials and New Energy Chemical Research Laboratory of MOE’s Research Center for Comprehensive Utilization and Clean Processing Engineering of Phosphorus Resources, has made a breakthrough in lithium-ion battery research, and published in the top-notch academic journal Advanced Science ( IF: 12.441 ) its research findings: "Suppressing Manganese Dissolution via Exposing Stable{111} Facets for High-Performance Lithium-Ion Oxide Cathode". The first author of the research paper is Yao Xiao, a class 2016 Ph.D. candidate in SCU School of Chemical Engineering. The corresponding authors are Professor Xiao-Dong Guo and Professor Yu-Guo Guo, a distinguished professor and research fellow of Sichuan University. SCU School of Chemical Engineering is the first work unit.

Being environmentally friendly, Lithium-ion batteries have the advantages of long life and high energy density. Therefore, they are the best chemical power sources so far. Lithium-ion batteries have become dominant in the 3C electronic field and have also shown a strong momentum in power batteries. Among them, the spinel-type LiMn2O4cathode material has been widely used and enjoyed a fairly good market share in the field of power batteries due to its simple synthesis process and cost efficiency. However, the LiMn2O4cathode material contains a large amount of Mn3+, which is prone to disproportionation, generates Mn2+ and dissolves in the electrolyte, causing corrosion of the cathode material surface. Simultaneously, the dissolved Mn2+is transferred to the surface of the graphite negative electrode by the concentration gradient and the electric field, and precipitates, which affects the formation of the SEI film on the surface of the negative electrode, resulting in serious deterioration of the battery performance especially at elevated temperature. Consequently, improving the stability of the surface structure of the positive electrode material and therefore inhibiting the manganese dissolution are keys to improving the performance of the cell of the spinel-type LiMn2O4cathode material.
Therefore, researchers of the team designed a high-performance lithium-ion battery cathode material with preferentially exposed stable {111} facets through the apparent geometrical structure design. At the same time, this cathode material also has a 3D hollow fusiform structure. On the one hand, the unique structure can shorten the transmission distance of Li+and greatly improve the rate performance. On the other hand, the structural strain and volume change generated during the charge-discharge process can also be buffered. Moreover, the preferentially exposed stable {111} facets could provide denser crystal lattice of manganese atoms, which can minimize interfacial side reactions between the cathodes and organic electrolytes, and is conducive to the formation of stable SEI, which remarkably improves the cycling stability of the material structure, thereby greatly improving the electrochemical properties of the material. The preferential facets design strategy proposed in this work provides guidelines for the modulation of the physicochemical properties of spinel-type cathode materials, which can be further extended to other research of cathode material systems, providing a new idea for the future development of high-performance lithium ion battery cathode materials.
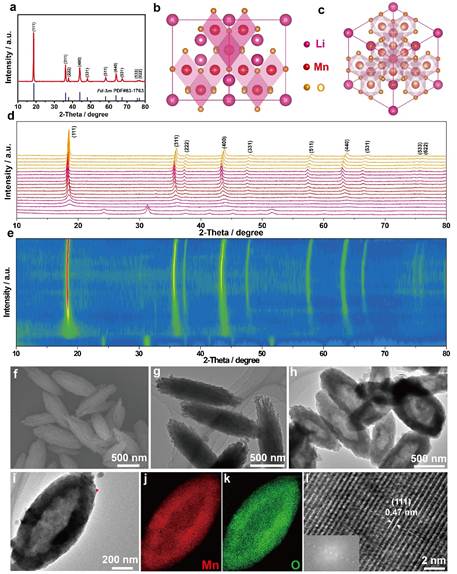
Figure 1. a) Powder XRD pattern of LMO-HF material. b,c) Fd-3m crystal structure viewed along the [110] and [111] crystallographic directions. d,e) In situ XRD patterns at different temperatures of precursors concerning LMO-HF cathode material and intensity contour maps concerning the evolution of the main characteristic diffraction peaks. f,g) SEM and TEM images of MnCO3precursor. h-l) TEM images, EDS mapping, and HR-TEM image of LMO-HF material at different magnifications (red dot stands for the position of HR-TEM image)

Figure 2. a,b) TEM and HR-TEM images of LMO-HF material with FFT pattern as inset. c,d) Enlarged HR-TEM images at different sites. e,f) TEM and HR-TEM images with FFT pattern as inset at different sites. g,h) Enlarged HR-TEM images at different sites. i,j) TEM and HR-TEM images with FFT pattern as inset at different sites. k,l) Enlarged image HR-TEM images at different sites.

Figure 3. a−f) Cross-sectional FIB-SEM images of LMO-HF material at different sites viewed from different angles. g−i) corresponding 3D reconstructed cross-sectional images at different state.

Figure 4. a,b) HAADF and ABF-STEM images of LMO-HF material viewed along the [110] crystallographic direction. c,d) corresponding enlarged HAADF and ABF-STEM images and model of atomic structure.

Figure 5. a) Galvanostatic charge/discharge curves versus specific capacity in the first cycle with a current density of 0.5 C at 25 °C. b) Cyclic voltammogram with a scan rate of 0.1 mV·s−1at 25 °C. c,d) Rate performance and corresponding galvanostatic charge/discharge curves versus specific capacity at various rates at 25 °C. e,f) Cyclic voltammograms at different scan rates and the plotting of peak current versus square root of the scan rate at different oxidation and reduction peaks at 25 °C. g,h) A single titration during GITT measurement and voltage with ohmic polarization in the first cycle during the whole charge process at 25 °C. i) Cycling performance during 1000 cycles with a current density of 1 C at 25 °C. j) Cycling performance during 500 cycles with a current density of 0.5 C at 60 °C.
